Global Viral Clearance Market Size, Share, Trends & Growth Forecast Report By Method, Application, End-User and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), Industry Analysis From 2025 To 2033.
Global Viral Clearance Market Size
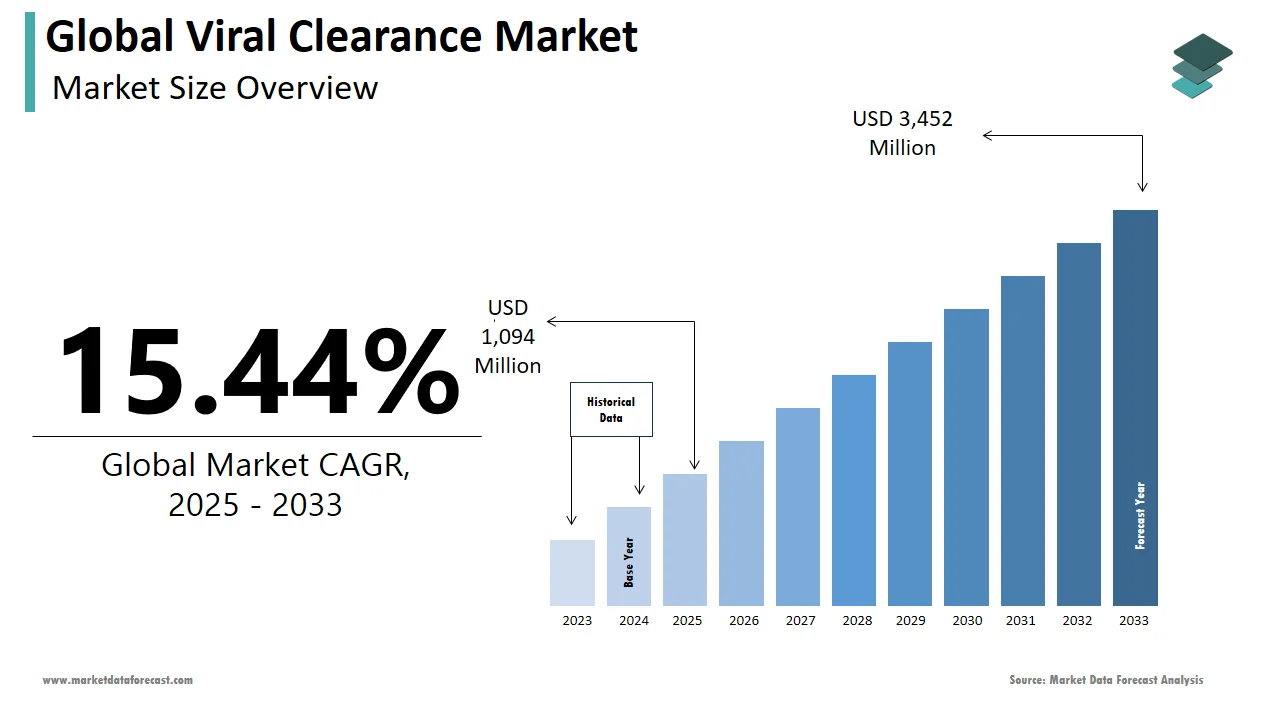
The size of the global viral clearance market was worth USD 948 million in 2024. The global market is anticipated to grow at a CAGR of 15.44% from 2025 to 2033 and be worth USD 3,452 million by 2033 from USD 1,094 million in 2025.
MARKET OVERVIEW
The growing prevalence of chronic diseases, growing approvals of biosimilars, and increasing technological advancements are primarily fuelling the development of the viral clearance market.
Increasing demand for efficient treatment methods and rising viral safety rules are likely to spike the growth of the viral clearance market. In addition, the expansion of biosimilar approval can improve patient access to care by increasing the number of medication procedures at a lower price. Furthermore, increasing healthcare expenditure, development of technological advancements, and new & enhanced therapies to treat the rise in many diseases, such as nanofiltration technology, and growing drug discoveries further contribute to the growth of the viral clearance market.
The rising number of new drugs, growing R&D investments and increasing government initiatives, rise in funding from government, public, and private bodies towards the pharmaceutical industry, rising geriatric population, increasing healthcare expenditure, and rising demand are other significant factors that are expected to boost the growth of the global viral clearance market. In addition, increased emphasis on quality assurance and quality control departments and growing penetration of specialty drugs is the opportunity for growth of the global market.
However, the time-consuming process of drug development and problems associated with the virus clearance process, such as protein crosslinking, virus aggregate formulation, and protein denaturation and degradation, is restraining the market growth during the forecast period. In addition, the rise in cost, the shortage of skilled professionals, and the increased degree of consolidation are further anticipated to challenge the development of the viral clearance market during the forecast period.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
15.44% |
|
Segments Covered |
By Method, Application, End-User, and Region. |
|
Various Analyses Covered |
Global, Regional, and country-level analysis; Segment-Level Analysis, DROC; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape; Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
Merck KGaA, Charles River, WuXi Biologics, Texcell, Vironova, Kedrion, Clean Cells, ViruSure GmbH, Sartorius AG, and Others. |
SEGMENTAL ANALYSIS
By Method Insights
Based on the method, the viral removal segment held the largest share of the viral clearance market in 2024. The factors driving the segment's growth are high acceptance of this method, developing R&D activities by biopharmaceutical companies, and increasing demand for new products like plasma proteins and gene therapy products. In addition, this segment may experience strong growth due to its efficiency and flexibility of the process.
By Application Insights
The recombinant proteins segment had a significant share of the viral clearance market in 2024. Applications such as proteomics, structural biology, and protein therapeutics require large-scale recombinant protein production. The major factors driving the segment's development are the increasing demand for biopharmaceuticals for treating chronic conditions such as diabetes and cancer and the implementation of advanced techniques.
By End-User Insights
Based on the end-user, the CRO segment is expected to witness a significant share in the global viral clearance market over the forecast period. Outsourcing drug discovery services to CRO services by pharmaceutical companies, start-ups, and small biotechnology companies and increasing investments in drug discovery are driving segment growth.
REGIONAL ANALYSIS
Geographically, North America led the viral clearance market worldwide in 2024 owing to the growth of pharmaceuticals, increased production of monoclonal antibodies, the growing support for biotechnology from various institutes, increased R&D investments, and accuracy and flexibility of methods in life sciences research. In addition, increasing disease prevalence, a growing number of drug approvals, and government support for medication development are some of the factors driving the viral clearance market in the U.S. In the United States alone, 6 in 10 adults suffer from a chronic disease, as per the Centers for Disease Control and Prevention (CDC) 2022. Every year, about USD 3.8 trillion spent on treating chronic disorders.
Europe is expected to follow the North American region to lead the viral clearance market worldwide during the forecast period due to the high investment in research and development, pharmaceuticals, and the development of advanced products.
The Asia Pacific region is forecasted to showcase the fastest CAGR in the global market during the forecast period due to an increase in the development and manufacturing of generics and rising government investments in medical research, increasing the possibility of cell culture contamination and the presence of CROs in the countries like China-Japan, and India. More than 15 million people between the ages of 35 and 69 years die because of chronic diseases, and 85% of premature deaths occur in developed and developing countries, as per statistics by the WHO. In addition, many clinical research services, mainly in China, are expected to drive market growth in the coming years.
TOP PLAYERS IN THIS MARKET
Merck KGaA, Charles River, WuXi Biologics, Texcell, Vironova, Kedrion, Clean Cells, ViruSure GmbH, and Sartorius AG are some of the noteworthy companies operating in the global viral clearance market profiled in this report.
RECENT HAPPENINGS IN THIS MARKET
- In 2021, WuXi Biologics announced a biologics-integrated innovation center that had been operated in Hangzhou, Zhejiang province, China.
- In 2022, Asahi Kasei Medical signed an agreement to acquire Bionova Scientific, LLC.
- In 2021, the WuXi Biologics Biosafety Testing Facility was granted its second EMA GMP Certificate. WuXi Biologics' compliance with global cGMP biosafety testing standards and regulatory guidelines is verified by this certificate.
- Bionique Testing Laboratories LLC, a global leader in mycoplasma testing services for the biologics and life sciences industries, revealed in 2021 that it had been entirely purchased by a subsidiary of Japanese healthcare pioneer Asahi Kasei Medical Co., Ltd.
MARKET SEGMENTATION
This research report on the global viral clearance market has been segmented and sub-segmented based on the type, end-user, and region.
By Method Insights
- Viral Removal
- Chromatography
- Nanofiltration
- Precipitation
- Viral Inactivation
By Application Insights
- Recombinant Proteins
- Tissue & Blood-Derived Products
- Cellular & Gene Therapy Products
- Stem Cell Products
- Vaccines & Therapeutics
By End-User Insights
- Biopharmaceuticals
- Contract Research Organizations (CROs)
- Academic Research Institutes
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
