Global Transdermal Patch Market Size, Share, Trends & Growth Forecast Report By Application, End User and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), Industry Analysis From 2025 To 2033.
Global Transdermal Patch Market Size
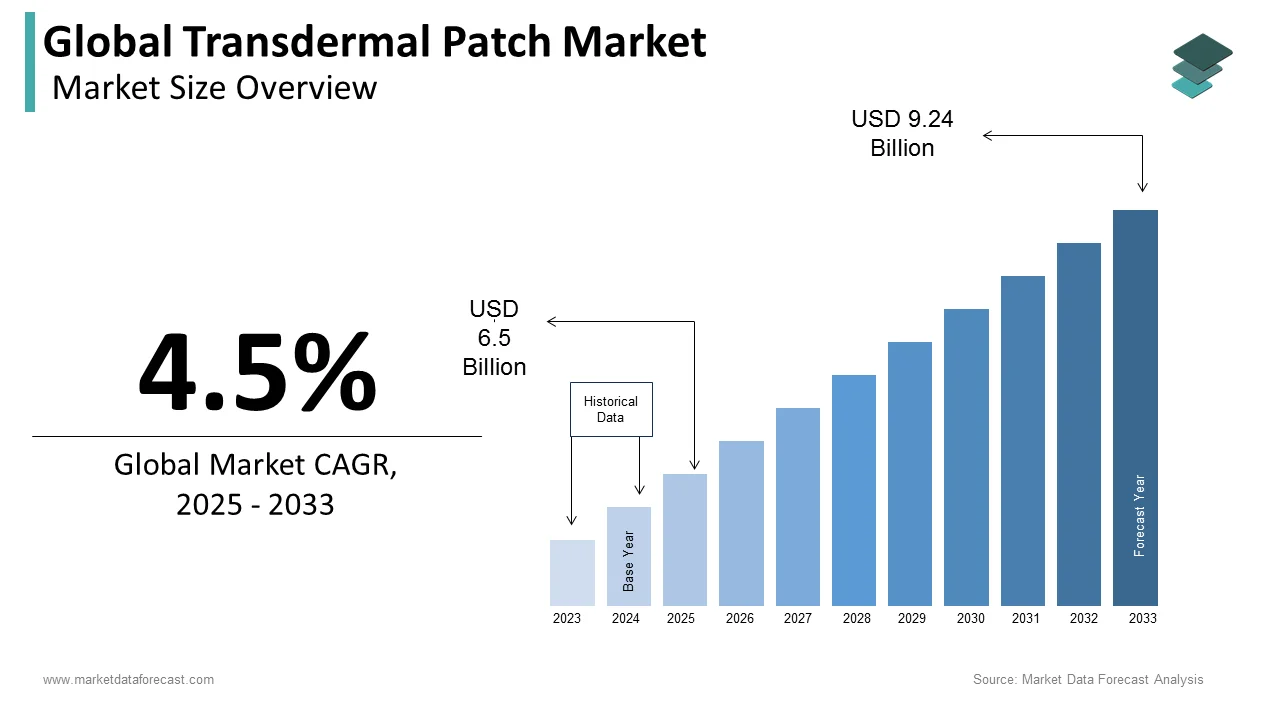
The global transdermal patch market was worth US$ 6.22 billion in 2024 and is anticipated to reach a valuation of US$ 9.24 billion by 2033 from US$ 6.5 billion in 2025, and it is predicted to register a CAGR of 4.5% during the forecast period 2025-2033.
Transdermal Patch is used to deliver a specific dose of medication through the skin directly into the bloodstream. A transdermal patch heals the injured area quickly with medicated adhesive tape. The medication dose is delivered into the bloodstream through the patch, which is placed on the skin. This differs from the other treatment procedures, like taking pills orally and the intravenous delivery process.
MARKET DRIVERS
Rising technological advancements in transdermal drug delivery systems, increasing non-invasive and painless medication administration, and increasing adoption of various strategies such as product innovation are majorly driving the global transdermal patch market growth.
The growing benefits of transdermal medicine over oral and absorbing medicines (medications for gastrointestinal toxicity) that cause nausea and vomiting are some of the key drivers driving the market's rise. Due to the simplicity of administration and the drug's sustained activity, the worldwide transdermal patches market is anticipated to expand significantly over the forecast period. Migraine, hormones, pain, cardiovascular illnesses, neurological disease, Parkinson’s disease, Alzheimer’s disease, strokes, HIV, osteoporosis, dermatology-related problems, restless leg syndrome therapies, and smoking cessation are all treated with these patches. Due to reduced dosage frequency, increased bioavailability, fewer side effects, and drug input cessation at any time by removing the patch, the transdermal route of drug administration is becoming more popular.Growing collaborations between pharmaceutical companies and drug delivery businesses and growing self-administration and home care medication delivery systems are further estimated to expand the market for transdermal patches during the forecast period. Increasing product launches and approvals, rising R&D investment for advancements in transdermal patches, mergers, and acquisitions as developmental strategies to maintain the competitive environment, rising beneficial government initiatives, and increased research are the driving factors for the transdermal patches market in coming years. The number of medicines administered trans-dermally increases because of advances in modern technology, including small-molecule hydrophobic pharmaceuticals, hydrophilic drugs, and macromolecules. The improved efficiency and usage of transdermal drug delivery systems (TDDS) resulting from new technological advancements have boosted their appeal and demand. Transdermal patch technologies are constantly being improved to make them more adaptable and popular in the United States. To strengthen and extend their presence for the growth of the transdermal patches market, major firms in the market are increasingly opting for acquisitions and collaborations.
MARKET RESTRAINTS
The growing difficulty of the skin to absorb a wide range of medicines and the increased risk of contact dermatitis or other skin diseases at the application site are among the key factors that will hinder market expansion. In addition, the market for transdermal patches is being hindered by stringent regulatory approval from government regulatory agencies. The market for transdermal patches is predicted to be further hampered by rising technological challenges such as skin irritation and permeability.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
4.5% |
|
Segments Covered |
By Application, End-User, and Region. |
|
Various Analyses Covered |
Global, Regional, and country-level analysis; Segment-Level Analysis, DROC; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape; Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
Hisamitsu Pharmaceutical (Japan), Mylan (US), UCB (Belgium), Novartis (Switzerland), and GlaxoSmithKline (UK). Boehringer Ingelheim (Germany), Johnson & Johnson (US), Endo International (Ireland), and Purdue Pharma (US)., and Others. |
SEGMENTAL ANALYSIS
By Application Insights
Based on application, the pain management segment had the major share of the global transdermal patch market in 2024 and is likely to continue the domination in the market during the forecast period owing to the growing incidence of chronic pain and increasing awareness demand for no or fewer side effects medication process.The cardiovascular diseases segment is expected to grow at a promising CAGR of 12% during the forecast period due to the growing usage of the systems for treating hypertension, congestive heart failure, and angina pectoris.
By End User Insights
Based on end-user, the homecare settings segment held the major share of the global market in 2024 and is predicted to grow rapidly during the forecast period owing to the rising demand for self-medication.On the other hand, the hospitals and clinics segment is expected to grow at a healthy rate during the forecast period. Growing priority on obtaining transdermal drug delivery systems among the clinics surges the segment growth globally. On the other hand, the hospital's segment plays an important role by offering a wide range of medical facilities to a huge patient population affected by different diseases, escalating the transdermal patch's growth globally.
REGIONAL ANALYSIS
Regionally, North America and Europe lead the market due to an increased focus on bioavailability products. North America is to continue the same flow in leading the highest shares of the market. The growth is primarily attributed to the growing healthcare expenditure, expiry of the license, heavy investments made by the important key players present in the region, and new key players who just stepped into the market growth globally. The US is leading the region with a significant share of encouraging healthcare schemes, and the rising patient count fosters the market. Canada followed the US in driving the market with quick growth in the region. Advanced manufacturing plants, high acceptable pace, and the assumption of progressive technologies escalate the region's market growth.
Europe is projected to follow North America in having the highest share in the transdermal patch market and is considered to have notable growth in the region over the period. An increase in the advent of medical tourism and the mounting occurrence of cases of cancer and diabetes impels the transdermal patch market's demand. The UK is to rule the transdermal patch market with a share of 12.2%. In addition, the surge in chronic diseases and the growing usage of transdermal drug patches for CVD are swelling the market globally.
The Asia Pacific is growing faster in recent years with the increasing geriatric population and cost-effective treatments. In addition, quickly developing healthcare infrastructure and fostering alertness in existing countries like China and India, and high research and development are majorly accelerating the market in this region.
The transdermal patch market in Latin America is projected to grow at a steady rate and be boosted by its increasing patient population base and formulation of new existing drug compounds.
The Middle East and Africa are elevated to have a slight inclination in the near future growth rate.
KEY MARKET PLAYERS
Companies playing an important role in the global transdermal patch market profiled in this report are Hisamitsu Pharmaceutical (Japan), Mylan (US), UCB (Belgium), Novartis (Switzerland), and GlaxoSmithKline (UK). Boehringer Ingelheim (Germany), Johnson & Johnson (US), Endo International (Ireland), and Purdue Pharma (US).
RECENT MARKET HAPPENINGS
-
In October 2019, Noven Pharmaceutical, a wholly-owned subsidiary of Hisamitsu Pharmaceutical Company, has revealed that the company has received U.S FDA approval for the transdermal patch asenapine (Secuado) and is the first approved patch for adults with schizophrenia.
-
In July 2018, UCB received approval from the China FDA for the approved neuro patched (rotigotine) to treat patients suffering from idiopathy. Patients are more advantageous from this strategy throughout the disease.
MARKET SEGMENTATION
This research report on the global transdermal patch market has been segmented and sub-segmented based on the application, end-user, and region.
By Application
- Pain Management
- Central Nervous System disorders
- Hormonal Applications
- Cardiovascular Diseases
- Others
By End User
- Home Care Settings
- Hospitals & Clinics
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
Frequently Asked Questions
What is the current size of the transdermal patch market?
The global transdermal patch market was worth USD 6.22 billion in 2024.
What factors are driving the growth of the transdermal patch market?
The growth of the transdermal patch market is being driven by factors such as the increasing prevalence of chronic diseases, the growing demand for non-invasive drug delivery methods, and the development of new technologies for the manufacture of transdermal patches.
What are the challenges faced by the transdermal patch market?
Some of the challenges faced by the transdermal patch market include the high cost of development and manufacturing, regulatory hurdles, and competition from other drug delivery methods.
What are the key players in the transdermal patch market?
Some of the key players in the transdermal patch market include Novartis AG, Johnson & Johnson, Mylan N.V., Teva Pharmaceutical Industries Ltd., and Hisamitsu Pharmaceutical Co., Inc.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
