Global Regulatory Information Management Market Size, Share, Trends & Growth Forecast Report By Type, Application and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), Industry Analysis From 2025 To 2033.
Global Regulatory Information Management Market Size
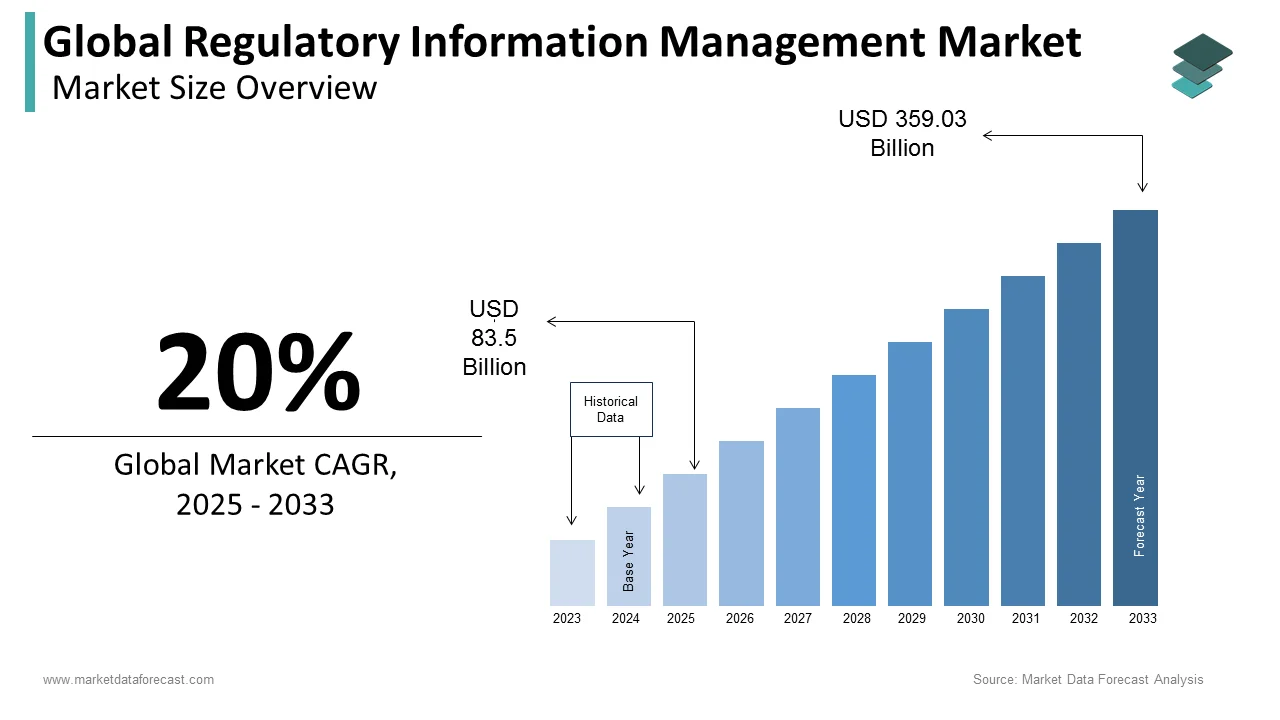
The global regulatory information management market was worth US$ 69.6 billion in 2024 and is anticipated to reach a valuation of US$ 359.03 billion by 2033 from US$ 83.5 billion in 2025, and it is predicted to register a CAGR of 20% during the forecast period 2025-2033.
The regulatory information management process includes many departments in an organization with proper planning. Regulatory information management allows tracking the product details and ensures the content is stored in electronic documents. This process makes it easy to publish applications and reminds the organizations of project submission dates.
MARKET DRIVERS
Growing regulatory reporting requirements and corporate financial imperatives drive a regulatory shift from a primarily document-based model to a data-driven paradigm. Managing structured and unstructured content and a multitude of metadata demands a new level of precision in fulfilling regulatory responsibilities provided by regulatory information management software. The world is driven by an ever-increasing demand for speed to market, making it difficult for brands to maintain pharmaceutical regulatory compliance. The unification of information enabled by effective regulatory information management software and benefits such as greater consistency and reduced unnecessary effort promote its implementation in verticals.
Organizations are increasingly striving to derive additional value from their product data as they realize that strategically important data could play a role in driving productivity, competition, and competitive differentiation. This drives regulatory information management software implementation, along with the growing importance of making products and regulatory information more shareable between and beyond specific functions. With the implementation of the new European requirements and the fact that other regions are considering adopting similar requirements, companies will have to send greater volumes of structured product information across demographics. Therefore, a unified regulatory information management capability is vital for collecting, linking, and shipping data, thus strengthening the broader supply chain with authentic and accessible product data.
Additionally, factors such as the growing prevalence of delivering effective applications and proper planning in the procedures, an increasing number of new players, and adopting the latest techniques to develop productivity are further propelling the growth of the global regulatory information management market. Furthermore, the rise in investments by public and private organizations and increasing support from the IT sector is expected to act favorably on the global regulatory information management market. The rapid transformation of life sciences compliance without losing momentum has made regulatory approval more complex, increasing the burden - both direct and indirect costs - of compliance for most organizations. With no alternative to compliance and the fact that the company’s approach to compliance directly impacts its competitiveness, the installation of regulatory information management software has increased dramatically.
MARKET RESTRAINTS
The rapidly evolving regulatory framework and product approval requirements continue to drive developers to keep abreast of the latest regulations and connect with market players to ensure the effectiveness of software for applications' error-free regulatory compliance process. These constant limitations make regulatory information management software less in demand by many businesses. In addition, the level of training required to use regulatory information management software has prevented its adoption, especially by small businesses wary of the additional costs associated with employee training.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
20% |
|
Segments Covered |
By Type, Application, and Region. |
|
Various Analyses Covered |
Global, Regional, & Country Level Analysis, Segment-Level Analysis; DROC, PESTLE Analysis; Porter’s Five Forces Analysis; Competitive Landscape; Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
Acuta LLC, Parexel, MasterControl, Sparta Systems, Veeva Systems, Computer Science Corp (CSC), Aris Global, Ennov, Amplexor, Samarind, and Dovel Technologies, and Others. |
SEGMENTAL ANALYSIS
By Type Insights
Based on type, the software segment is predicted to hold a significant share of the global regulatory information management market during the forecast period. A rise in the development of new technologies is fuelling the demand for this segment. In addition, the software is more appropriate for claims in industries like healthcare and life sciences, bolstering the segment growth in the market globally. Moreover, factors like modifications in administrative policies in companies like biotechnology, pharmaceuticals, and clinical research are likely to develop the development of the market during the period globally.
The services segment is forecasted to register a healthy CAGR during the forecast period due to the rise in a considerable volume of data gathered and support from regular bodies like the U.S FDA, European agencies, and other agencies to key market players.
By Application Insights
Based on the application, the pharmaceutical application is predicted to occupy a leading share in the global market during the forecast period due to the rise in the demand for the effective delivery of drugs.
REGIONAL ANALYSIS
Regionally, North America and Europe have had significant shares for the past few years. North America and Europe are accounted to lead the highest shares of the market by increasing capital income and rising investments in the research institutes. The Asia Pacific is anticipated to have the highest growth rate in the coming years. The Middle East and Africa are to have substantial growth opportunities in the near future. The regulatory information management market in Latin America is supposed to be in the developing stage. It is projected to have a substantial market grant during the estimated period. The surge in the need to decrease human-made errors in administrative processes and simple admittance globally fuels this region's market.
The Middle East & Africa is believed to have an evolving share in the regulatory information management market globally during the forecast period. In addition, the fostering requirement for the real-time observance of regulations in the clinical trial sector fosters market growth.
KEY MARKET PLAYERS
Companies dominating the global regulatory information management market profiled in this report are Acuta LLC, Parexel, MasterControl, Sparta Systems, Veeva Systems, Computer Science Corp (CSC), Aris Global, Ennov, Amplexor, Samarind, and Dovel Technologies., and Others.
MARKET SEGMENTATION
This research report on the global regulatory information management market has been segmented and sub-segmented based on the type, application, and region.
By Type
- Software
- Services
By Application
- Pharmaceutical Industry
- Biotechnology
- Clinical research organizations
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
