Global Progressive Ataxia and Weakness Disorders Market Size, Share, Trends & Growth Forecast Report – Segmented By Disorder Type (Progressive Ataxia and Progressive Weakness Disorders), Treatment (Pharmacological Therapies and Rehabilitation Therapies) and Region (North America, Europe, Asia Pacific, Latin America, Middle East and Africa) – Industry Analysis (2024 to 2029)
Global Progressive Ataxia and Weakness Disorders Market Size (2024 to 2029)
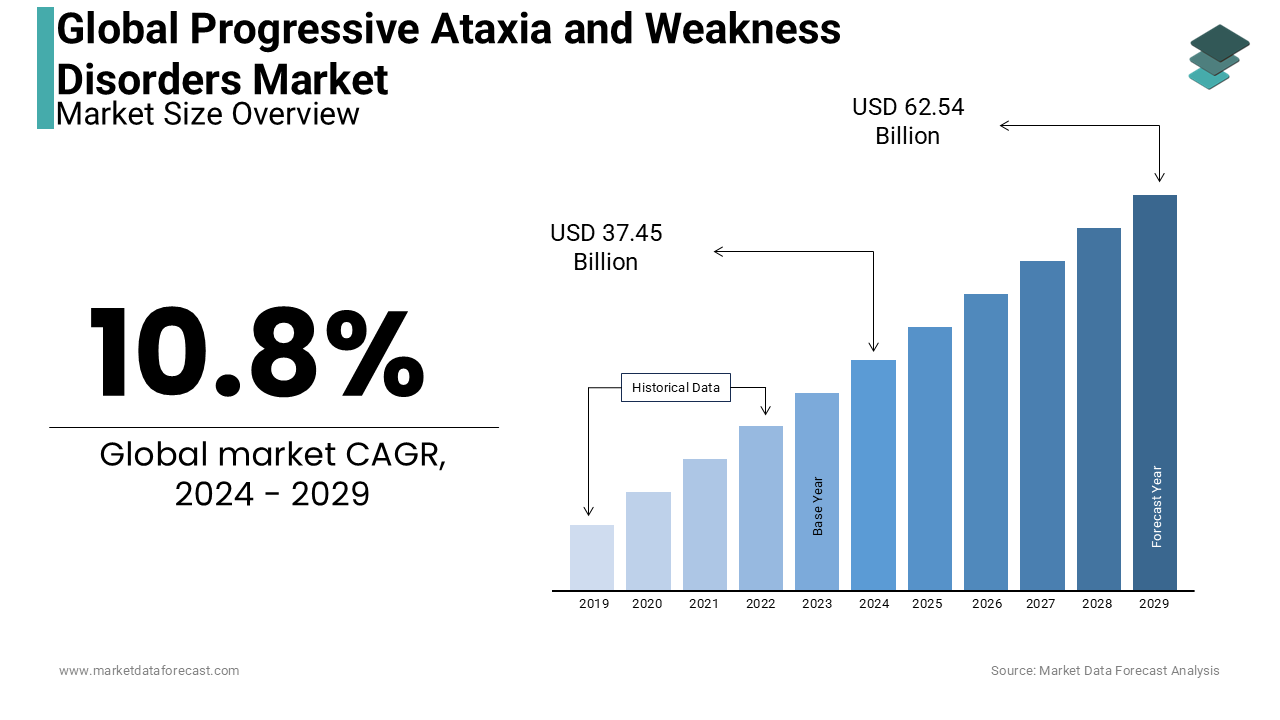
The size of the global progressive ataxia and weakness disorders market was valued at USD 33.8 billion in 2023 and the global market size is anticipated to reach USD 62.54 billion by 2029 from USD 37.45 billion in 2024, growing at a CAGR of 10.8% from 2024 to 2029.
Current Scenario of the Global Progressive Ataxia and Weakness Disorders Market
The treatment of syndromes of progressive ataxia and weakness disorders market is gradually moving forward with multiple clinical trials and the development of treatment and therapies for spinocerebellar ataxia 4 (SCA4), Friedreich ataxia, weakness and sensory deficits. Moreover, the market is also experiencing an increase in incidences of neurological disorders augmenting the growth rate by persuading industry players to invest more in the launch of new remedies of treatments. Similarly, because of legal compliance communications, Biohaven made a fresh protocol to evaluate the efficiency of troriluzole following three years of therapy in SCA. This new guideline, BHV4157-206-RWE (NCT06529146), utilises RWE-real world evidence (RWE) of strength and efficacy through RWD-real world data origins to investigate the therapy implications of troriluzole in SCA after a period of 3 years of treatment against those of external control participants gathered from the United States SCA Natural History cohort (CRC-SCA) and such developments are contributing to the global market growth.
MARKET DRIVERS
The rising frequency of neurological disorders is driving the progressive ataxia and weakness disorders market growth.
An important new study recently published stated that over 3 billion individuals around the world were suffering from a neurological condition and cited the excessive consumption of drugs and alcohol as the primary reason. The demand for the treatment market for progressive ataxia and weakness disorders is increasing exponentially in low and middle-income countries, which is because more than 80% of neurological fatalities and deterioration of well-being happen in low- and middle-income nations and availability of treatment differs broadly. This factor aids global market growth. In addition, developed countries have up to 70-fold higher numbers of neurological professionals per 100,000 individuals compared to low- and middle-income nations.
Technological advancements in the healthcare industry further boost the growth rate of the global market.
In addition, technological breakthroughs and recent product releases have supported the market progress. Advancements in potential treatment are fuelling the demand for the treatment of syndromes of progressive ataxia. Presently in the industry, there is no remedy for Spinocerebellar ataxia type 6 called SCA6, however, with the latest breakthrough by McGill University researchers which was published in March 2024 the market is expected to see an upward trend in market growth. As per the latest discovery, they were able to exhibit that the development of the disease is most probably because of the injured mitochondria in the cells part of the cerebellum.
MARKET RESTRAINTS
The difficulty and complications of the disorders are a major restraint to the progressive ataxia and weakness disorders market growth.
The lack of enough latest data, research, or findings is derailing the expansion of the global market. In addition, according to an investigation, to correlate the detected variants with their clinical characteristics, it is necessary to plan or devise genotyping-directed molecular studies. Its absence also affects the industry’s progress. For, even while acquiring a positive discovery from Whole-exome sequencing (WES), the physician is still required to correlate clinical phenotypes and the detected variations. Clinical problems persist after getting a negative or unidentified WES, affecting the growth of the market.
MARKET OPPORTUNITIES
The discovery of genetic diversity or variation that causes spinocerebellar ataxia 4 (SCA4) is expected to open new avenues for the progressive ataxia and weakness disorders market. This acts as a response to households and presents the way to future treatments. The researchers utilized a newly developed sophisticated sequencing technology and studied the DNA of unaffected and affected individuals from many Utah households. Apart from this, market expansion is also likely to happen in the area of SCA2, which is also meddling with the recycling of protein. The investigators are currently examining a highly possible treatment for SCA2 in clinical trials, and the parallels between the two conditions increase the potential for the therapy to be advantageous for SCA4 patients as well. Several unique strategies are currently being advanced to detect or determine potential SCA therapeutics that will push the market growth rate forward.
MARKET CHALLENGES
Insufficient treatment choices, along with financial and legal barriers, are hindering the treatment of syndromes of progressive ataxia and weakness disorders market. The lack of greater targeted remedies and disease-modifying therapies for ataxia obstructs industry development and patient results. Besides this, exorbitant research and development expenditures along with regulatory impediments to the approval of orphan drugs, could discourage the inflow of investments for treatment development in this market.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 to 2029 |
|
Base Year |
2023 |
|
Forecast Period |
2024 to 2029 |
|
CAGR |
10.8% |
|
Segments Covered |
By Disorder Type, Treatment Type and Region |
|
Various Analyses Covered |
Global, Regional and Country Level Analysis; Segment-Level Analysis; DROC; PESTLE Analysis; Porter’s Five Forces Analysis; Competitive Landscape; Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
Novartis AG, Pfizer Inc., Sanofi, Roche Holding Ltd., Teva Pharmaceuticals, American Regent, Abbot Laboratories, Biogen Idec., Cadila Healthcare, Dr. Reddy Laboratories, Baxter International, Bristol Myers Squibb and Eli Lilliy |
SEGMENTAL ANALYSIS
Global Progressive Ataxia and Weakness Disorders Market Analysis By Disorder Type
The Friedreich ataxia segment is likely to account for the major share of the global market during the forecast period. Friedreich ataxia is believed to be the most prevailing disorder under the treatment of syndromes of progressive ataxia and weakness disorders market. The growing incidence of human papillomavirus infections traceable to the non-utilization of condoms or dental dams while indulging in sex is aiding segmental growth. Apart from this, the increasing frequency of various related risk elements, like a poor immune system, many romantic partners, and participating in sexual relations at a young age, is also promoting segmental expansion.
Global Progressive Ataxia and Weakness Disorders Market Analysis By Treatment
The small molecule drugs segment led the market in 2023 and is expected to continue the dominating trend throughout the forecast period. The small molecule drugs sub-segment is leading the market in the treatment of syndromes of progressive ataxia and weakness disorders. The segment’s market growth can be credited to the effectiveness and targeted action, the presence of an established industry, focused current research and development, favorable legal approvals and drug repurposing, and emphasis on a patient-centric approach.
REGIONAL ANALYSIS
North America is anticipated to remain dominant in the global market.
North America is spearheading the treatment of syndromes of progressive ataxia and weakness disorders market. It is projected that less than 5000 people in the United States have Spinocerebellar ataxia type 6, which is the consequence of genetic changes or modifications in the cerebellum. Further, in the ataxia domain with modern and state-of-the-art medical infrastructure and substantial funding for research R&D projects and measures are other key factors accelerating the region’s market share in the discovery of new treatments. Also, the availability of specialized therapies and supportive and caring patient advocacy communities add to the expansion of market size in this region.
Europe is likely to show a healthy CAGR in the global market during the forecast period.
The growth of the European market is majorly driven by the rising cognizance of rare neurological disorders and joint research projects. Moreover, strict legal criteria ensure quality care and encourage the region’s market share. According to a 2024 study, in Europe, SCA3 is the most regular spinocerebellar ataxia in total. Its prevalence is exceptionally higher in Portugal and after that Germany, then France and the Netherlands. Nobody or few instances were depicted in Norway, Finland, Serbia, Poland, Russia and Italy. SCA2 and SCA1 worldwide exhibited similar incidences and are more common in France, Serbia, Poland, the United Kingdom and Italy.
KEY PLAYERS IN THE GLOBAL PROGRESSIVE ATAXIA AND WEAKNESS DISORDERS MARKET
Companies that play a notable role in the global progressive ataxia and weakness disorders market include Novartis AG, Pfizer Inc., Sanofi, Roche Holding Ltd., Teva Pharmaceuticals, American Regent, Abbot Laboratories, Biogen Idec., Cadila Healthcare, Dr. Reddy Laboratories, Baxter International, Bristol Myers Squibb and Eli Liliy.
RECENT HAPPENINGS IN THE GLOBAL MARKET
- In April 2024, a multinational study was published which, for the first time, decisively identified the variance in genetics that leads to SCA4, i.e., a progressive disease called spinocerebellar ataxia 4 (SCA4). This international research was headed by Stefan Pulst and K. Pattie Figueroa, both are part of the Spencer Fox Eccles School of Medicine under the University of Utah.
DETAILED SEGMENTATION OF THE GLOBAL PROGRESSIVE ATAXIA AND WEAKNESS DISORDERS MARKET INCLUDED IN THIS REPORT
This research report on the global progressive ataxia and weakness disorders market has been segmented and sub-segmented by disorder type, treatment type and region.
By Disorder Type
- Progressive Ataxia
- Spinocerebellar Ataxias (SCAs)
- Friedreich's Ataxia
- Multiple System Atrophy
- Episodic Ataxias
- Progressive Weakness Disorders
- Amyotrophic Lateral Sclerosis (ALS)
- Spinal Muscular Atrophy (SMA)
- Charcot-Marie-Tooth Disease
- Guillain-Barré Syndrome
By Treatment
- Pharmacological Therapies
- Small Molecule Drugs
- Monoclonal Antibodies
- Enzyme Inhibitors
- Rehabilitation Therapies
- Physical Therapy
- Occupational Therapy
- Speech Therapy
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Frequently Asked Questions
How big is the global progressive ataxia and weakness disorders market?
The size of the global progressive ataxia and weakness disorders market was worth USD 33.8 bn in 2023.
Which region is leading the progressive ataxia and weakness disorders market at present?
North America is presently playing the dominating role in the global progressive ataxia and weakness disorders market.
Who are the major companies in the global progressive ataxia and weakness disorders market?
Novartis AG, Pfizer Inc., Sanofi, Roche Holding Ltd., Teva Pharmaceuticals, American Regent, Abbot Laboratories, Biogen Idec., Cadila Healthcare, Dr. Reddy Laboratories, Baxter International, Bristol Myers Squibb and Eli Lilliy are some of the noteworthy companies in the global market.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
