North America Esoteric Testing Market Research Report – Segmented By Type ( Oncology , Genetic Tests ) and Country (The U.S., Canada and Rest of North America) - Industry Analysis, Size, Share, Growth, Trends, & Forecasts 2025 to 2033.
North America Esoteric Testing Market Size
The North America Esoteric Testing Market Size was valued at USD 11.18 billion in 2024. The North America Esoteric Testing Market size is expected to have 7.96 % CAGR from 2025 to 2033 and be worth USD 22.29 billion by 2033 from USD 12.08 billion in 2025.
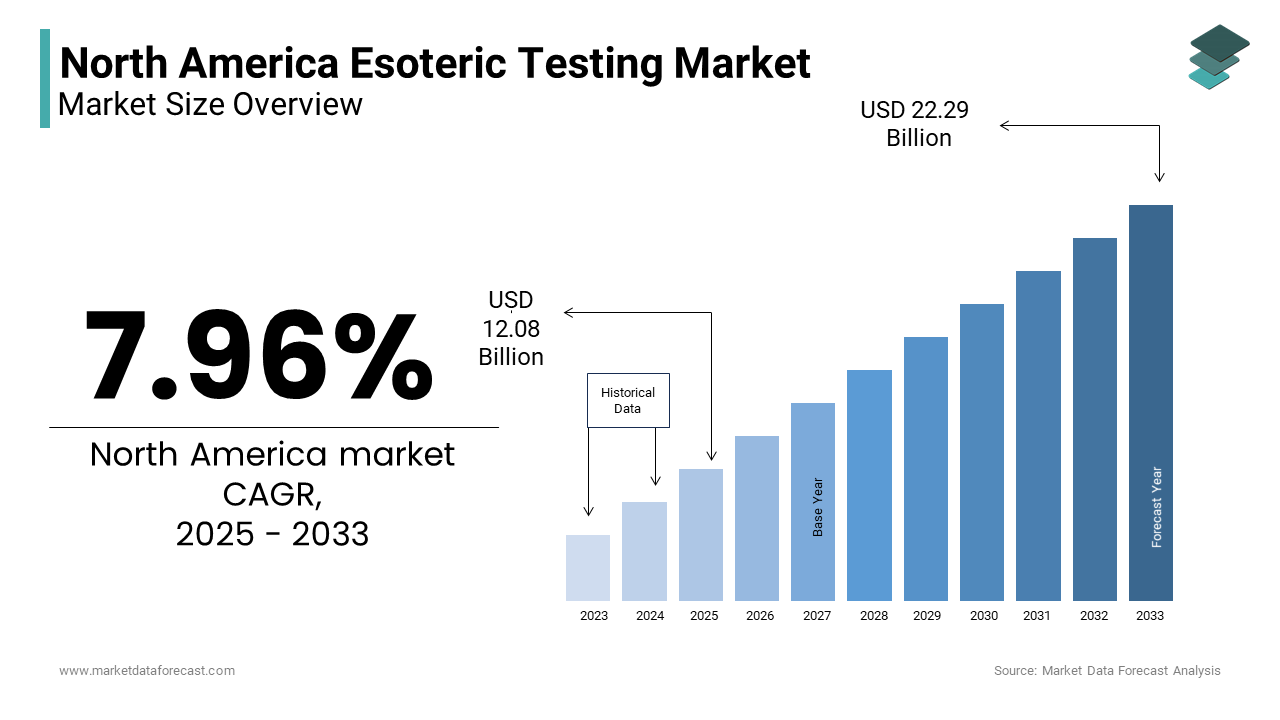
The North America esoteric testing market has emerged as a pivotal segment within the broader diagnostics industry, driven by its specialized focus on rare and complex diseases. Also, the United States accounts for the largest share and is attributed to its robust R&D ecosystem and widespread adoption of precision medicine. Canada follows, leveraging government initiatives to enhance diagnostic capabilities. A key factor shaping the regional scenario is the rising burden of cancer, with the American Cancer Society estimating over 1.9 million new cases in 2023 alone. This exhibits the necessity for oncology-specific esoteric tests, which form a significant portion of the market. Furthermore, advancements in genomic technologies have bolstered diagnostic accuracy, fostering trust among healthcare providers. Public-private partnerships, such as those between the NIH and private firms, have also propelled innovation, creating a conducive environment for market expansion.
MARKET DRIVERS
Increasing Prevalence of Chronic Diseases
Chronic diseases remain a dominant driver of the esoteric testing market in North America, with cardiovascular disorders, diabetes, and autoimmune conditions showing alarming growth rates. According to the Centers for Disease Control and Prevention, chronic diseases account for a significant portion of all deaths in the United States. This escalating burden necessitates precise diagnostic tools, propelling demand for specialized tests. For instance, autoimmune diseases like rheumatoid arthritis affect over 1.3 million Americans, as reported by the Arthritis Foundation. These conditions require frequent monitoring through esoteric assays, driving market revenue. Additionally, the rise in obesity-linked complications has intensified the need for endocrinology-based tests, further amplifying market growth.
Advancements in Molecular Diagnostics
Technological breakthroughs in molecular diagnostics represent another critical growth driver. Next-Generation Sequencing (NGS) and Polymerase Chain Reaction (PCR) technologies have revolutionized disease detection, enabling early intervention and personalized treatment plans. Like, NGS utilization has increased in the past few years, primarily due to its application in oncology and genetic disorders. The integration of AI and machine learning into diagnostic workflows has further enhanced test accuracy and efficiency. For example, liquid biopsy tests, which detect cancer biomarkers in blood samples, have gained traction. These innovations not only improve patient outcomes but also reduce long-term healthcare costs, making them indispensable in modern medicine.
MARKET RESTRAINTS
High Costs Associated with Esoteric Tests
One of the primary restraints hindering the growth of the esoteric testing market is the high cost associated with these specialized procedures. Advanced technologies like NGS and mass spectrometry require substantial investment in equipment and skilled personnel, translating into elevated testing fees. Such expenses often deter patients and healthcare providers, particularly in regions with limited insurance coverage. For instance, Medicaid reimbursement policies in several states do not adequately cover these tests, leaving patients to bear the financial burden. This affordability issue restricts market penetration, especially among underserved populations.
Regulatory Challenges and Compliance Issues
Stringent regulatory frameworks pose another significant challenge to market participants. Agencies like the FDA and CLIA enforce rigorous standards for test validation and quality assurance, which can delay product launches. As per insights from the American Clinical Laboratory Association, compliance with these regulations requires extensive documentation and validation studies, increasing operational costs. Moreover, inconsistencies in state-level regulations create additional hurdles for companies operating across multiple jurisdictions. For example, California mandates separate licensure for laboratories, adding layers of complexity. These regulatory barriers not only impede innovation but also discourage smaller players from entering the market, thereby limiting competition and stifling growth potential.
MARKET OPPORTUNITIES
Growing Adoption of Personalized Medicine
Personalized medicine represents a transformative opportunity for the esoteric testing market, driven by its ability to tailor treatments based on individual genetic profiles. This trend is particularly evident in oncology, where targeted therapies depend heavily on companion diagnostics. For instance, HER2 testing for breast cancer has become standard practice, with substantial number of tests conducted annually in the U.S. alone. The integration of AI-driven analytics further enhances the predictive power of these tests, enabling more accurate prognoses. As healthcare systems increasingly prioritize value-based care, the demand for personalized solutions is expected to surge, creating lucrative opportunities for market players.
Expansion of Telehealth Services
The rapid expansion of telehealth services presents another significant growth avenue for the esoteric testing market. Similarly, telehealth adoption surged notably during the pandemic, with sustained usage levels post-pandemic. Remote consultations facilitate access to specialized diagnostics, particularly for rural and underserved populations. For example, virtual platforms enable physicians to order esoteric tests directly from centralized labs, streamlining workflows and reducing turnaround times. This model not only improves patient convenience but also expands the customer base for testing providers. Furthermore, collaborations between telehealth companies and diagnostic labs are fostering innovation, such as home collection kits for blood and urine samples. These initiatives are expected to drive market expansion significantly over the next decade.
MARKET CHALLENGES
Limited Awareness Among Healthcare Providers
A persistent challenge facing the esoteric testing market is the limited awareness among healthcare providers regarding the availability and benefits of these specialized tests. Many clinicians remain unfamiliar with emerging technologies like liquid biopsies or advanced autoimmune panels, leading to underutilization. A significant percentage of primary care physicians lack comprehensive knowledge about esoteric diagnostics. This knowledge gap is particularly pronounced in rural areas, where continuing education resources are scarce. Consequently, patients may miss out on timely diagnoses, exacerbating disease progression.
Ethical Concerns Surrounding Genetic Testing
Ethical concerns surrounding genetic testing constitute another pressing challenge for the market. The potential misuse of genetic data, including discrimination by employers or insurers, has sparked public apprehension. As per findings from the Genetics and Public Policy Center, over 70% of Americans express privacy-related fears when considering genetic testing. These concerns are compounded by ambiguities in existing legislation, such as the Genetic Information Nondiscrimination Act (GINA), which offers limited protection beyond employment and health insurance contexts. Furthermore, debates around informed consent and data ownership have raised ethical dilemmas, deterring some individuals from undergoing testing. Addressing these issues necessitates robust policy reforms and transparent communication strategies to build public trust and ensure equitable access to these life-saving tools.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
7.96 % |
|
Segments Covered |
By Type and Country. |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis; DROC, PESTLE Analysis, Porter's Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Country Covered |
The U.S., Canada and Rest of North America |
|
Market Leader Profiled |
LabCorp (US) , Quest Diagnostics (US) , H.U. Group Holdings (Japan) |
SEGMENTAL ANALYSIS
By Type Insights
The oncology segment dominated the North America esoteric testing market by commanding a 35.7% market share in 2024. This growth is credited to the rising incidence of cancer, coupled with the increasing adoption of precision medicine. The American Cancer Society estimates that one in three individuals will be diagnosed with cancer during their lifetime, underscoring the critical role of oncology tests in early detection and treatment planning. Key drivers include advancements in biomarker identification, which enable tailored therapies for conditions like breast, lung, and colorectal cancers. Additionally, liquid biopsies offer non-invasive alternatives to traditional tissue biopsies, further fueling demand.
The genetic tests segment is projected to grow at the fastest CAGR of 12.5% from 2025 to 2033. This rapid expansion is fueled by advancements in genomics and the increasing emphasis on preventive healthcare. The Human Genome Project's legacy has paved the way for affordable sequencing technologies, reducing costs by over 99% since 2001. Today, whole-genome sequencing costs less, making it accessible for clinical applications. Genetic tests are instrumental in identifying predispositions to hereditary conditions such as cystic fibrosis and Huntington’s disease, with significant number of tests available globally. The integration of AI and big data analytics further enhances diagnostic accuracy, enabling early interventions. Additionally, the growing popularity of direct-to-consumer genetic testing kits, reflects shifting consumer preferences toward proactive health management.
COUNTRY LEVEL ANALYSIS
The United States was the epicenter of the North America esoteric testing market by commanding an 85.5% share of the region's total revenue in 2024. This leading position in the market is rooted in a confluence of factors, including unparalleled healthcare infrastructure, substantial federal funding for research and development, and a growing prevalence of chronic diseases. According to the National Institutes of Health (NIH), federal funding for biomedical research exceeded USD 45 billion in 2023, underscoring the nation's commitment to advancing diagnostic technologies. Chronic illnesses such as cancer, diabetes, and autoimmune disorders are pervasive, with cardiovascular diseases alone affecting a significant percentage of Americans. These conditions necessitate specialized diagnostic tools, propelling demand for esoteric tests, particularly in oncology, endocrinology, and neurology. Additionally, favorable reimbursement policies have played a pivotal role in enhancing accessibility. For instance, Medicare covers certain genetic tests, enabling patients to benefit from cutting-edge diagnostics without prohibitive costs. The presence of major diagnostic companies like Quest Diagnostics, Labcorp, and BioReference Laboratories further cements the U.S.'s leadership position. These companies not only invest heavily in R&D but also collaborate with academic institutions and pharmaceutical firms to drive innovation.

Canada captured a modest yet significant share of the North America esoteric testing market and is driven by government-led healthcare initiatives and a focus on precision medicine. The Canadian healthcare system, characterized by universal coverage, ensures equitable access to diagnostic services, fostering trust among patients and healthcare providers. A notable example is the Pan-Canadian Health Genomics Strategy, which aims to integrate genomics into routine clinical practice, thereby accelerating the adoption of advanced testing methods. Initiatives like the Canadian Partnership Against Cancer have further promoted nationwide access to oncology diagnostics, addressing disparities in rural and remote areas. Collaborations with international research institutions have also facilitated knowledge exchange, enhancing the quality and reliability of esoteric tests. Despite these advancements, geographic barriers pose a challenge, as the vast and sparsely populated regions complicate logistics and service delivery.
The "Rest of North America," primarily comprising Mexico, is smaller in comparison to the U.S. and Canada. This region is poised for growth due to increasing healthcare investments and rising awareness about precision medicine. Moreover, the prevalence of infectious diseases, such as dengue and tuberculosis, has heightened the demand for specialized tests, particularly in immunology and microbiology. Moreover, collaborations with multinational corporations have introduced advanced technologies to the region, bridging gaps in diagnostic capabilities. For instance, partnerships with U.S.-based firms have enabled local labs to adopt next-generation sequencing and flow cytometry, improving test accuracy and efficiency. However, challenges such as limited insurance coverage and socioeconomic disparities hinder widespread adoption.
KEY MARKET PLAYERS AND COMPETITIVE LANDSCAPE
Companies playing a prominent role in the North America Esoteric Testing Market are LabCorp (US) , Quest Diagnostics (US) , H.U. Group Holdings (Japan) , Sonic Healthcare (Australia) , OPKO Health Inc. (US).
The North America esoteric testing market is characterized by intense competition, with established players vying for technological superiority and market share. Companies like Quest Diagnostics, Labcorp, and BioReference Laboratories dominate the landscape, leveraging their extensive resources and expertise to maintain leadership positions. The presence of high entry barriers, including substantial capital requirements and stringent regulatory standards, limits the participation of new entrants. However, niche players are increasingly gaining traction by offering specialized services tailored to underserved segments. For instance, smaller firms have capitalized on the growing demand for direct-to-consumer genetic testing, introducing affordable and accessible solutions that cater to shifting consumer preferences. Collaborations and alliances further intensify rivalry, as companies seek to expand their capabilities and tap into emerging opportunities. Strategic partnerships with pharmaceutical firms, academic institutions, and technology providers have become commonplace, enabling market participants to co-develop innovative diagnostics and stay ahead of the competition. The integration of AI and big data analytics has emerged as a key differentiator, enhancing diagnostic accuracy and operational efficiency.
Top Players in the Market
Quest Diagnostics
Quest Diagnostics is a global leader in diagnostic information services. The company specializes in oncology, neurology, and genetic testing, offering over 3,000 specialized assays designed to address complex medical conditions. Its proprietary platforms, such as Quanum, integrate AI-driven analytics to enhance diagnostic accuracy and streamline workflows for healthcare providers. This innovation not only improves patient outcomes but also reduces long-term healthcare costs by enabling timely interventions. Furthermore, the company's strategic acquisitions, such as Pack Health in 2024, underscore its commitment to expanding its digital health ecosystem and enhancing patient engagement. With a robust network of laboratories and partnerships with pharmaceutical giants, Quest Diagnostics continues to set benchmarks in the industry.
Labcorp
Labcorp is excelling in autoimmune and infectious disease testing. Labcorp's extensive research initiatives have led to the development of novel biomarkers for rare diseases, addressing unmet medical needs. For instance, its collaboration with Moderna resulted in the co-development of companion diagnostics for mRNA-based therapies, aligning with the growing trend toward personalized medicine. Additionally, Labcorp's investment in automation and artificial intelligence has enhanced operational efficiency, reducing turnaround times and improving test reliability.
BioReference Laboratories
BioReference Laboratories renowned for its innovative solutions in endocrinology and genetic testing. The company's partnership with big players in recent years exemplifies its commitment to advancing precision medicine through the development of novel biomarkers for autoimmune diseases. BioReference's proprietary platform, GeneDx, offers comprehensive genetic testing services, enabling early detection of hereditary conditions such as cystic fibrosis and Huntington’s disease. The company's low-cost genetic testing kits, introduced in 2023, have gained popularity among underserved populations, democratizing access to advanced diagnostics. Furthermore, BioReference's integration of AI-driven analytics into its workflows has improved diagnostic accuracy, enabling more precise prognoses. By focusing on affordability, accessibility, and technological innovation, BioReference Laboratories continues to carve out a distinct niche in the competitive landscape.
Top Strategies Used by Key Players
Key players in the North America esoteric testing market employ a multifaceted approach to maintain competitiveness and drive growth. Strategic mergers and acquisitions are a cornerstone of their expansion strategies, enabling them to broaden their service portfolios and enhance technological capabilities. For instance, Quest Diagnostics' acquisition of Pack Health in April 2024 strengthened its digital health offerings, allowing the company to provide end-to-end solutions for patient engagement and care management. Collaborations with pharmaceutical companies and research institutions are another critical strategy. Investments in R&D remain a top priority, with companies like BioReference Laboratories launching affordable genetic testing kits to address socioeconomic disparities in healthcare access. Additionally, the integration of AI and machine learning into diagnostic workflows has become a focal point, enhancing test accuracy and operational efficiency. These strategies collectively enable market leaders to stay ahead of the curve, driving innovation and meeting evolving patient needs.
RECENT HAPPENINGS IN THE MARKET
- In April 2024, Quest Diagnostics launched a new AI-driven platform to streamline test result interpretation, enhancing diagnostic precision and enabling healthcare providers to deliver more accurate prognoses.
- In June 2023, Labcorp acquired Chiltern International, expanding its clinical trial services portfolio and reinforcing its position as a leader in companion diagnostics for emerging therapies.
- In February 2024, BioReference Laboratories partnered with Pfizer to co-develop novel biomarkers for autoimmune diseases, addressing unmet medical needs and advancing precision medicine.
- In September 2023, Invitae introduced a low-cost genetic testing kit targeting underserved populations, democratizing access to advanced diagnostics and addressing socioeconomic disparities in healthcare.
- In January 2024, Mayo Clinic unveiled a next-generation sequencing panel for rare genetic disorders, improving detection rates and enabling early interventions for patients with hereditary conditions.
MARKET SEGMENTATION
This research report on the north america esoteric testing market has been segmented and sub-segmented into the following.
By Type
- Oncology
- Genetic Tests
By Country
- The U.S.
- Canada
- Rest of North America.
Frequently Asked Questions
Which countries dominate the North America Esoteric Testing Market ?
The United States holds the largest market share, followed by Canada. The U.S. is dominant due to its advanced healthcare infrastructure, high R&D investments, and the presence of major diagnostic companies.
What is the North America Esoteric Testing Market?
The North America Esoteric Testing Market refers to a specialized segment of the diagnostic testing industry focused on complex, rare, and highly specialized tests that are not routinely performed in standard clinical laboratories.
How does regulatory policy impact esoteric testing in North America?
Regulatory bodies like the FDA and CMS (through CLIA regulations) oversee test quality and validation. Compliance is critical for market entry and sustained operations.
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
