North America Endoscope Reprocessing Market Size, Share, Trends & Growth Forecast Report By Product, End User and Country (United States, Canada, Mexico, Rest of North America) – Industry Analysis From 2025 to 2033.
North America Endoscope Reprocessing Market Size
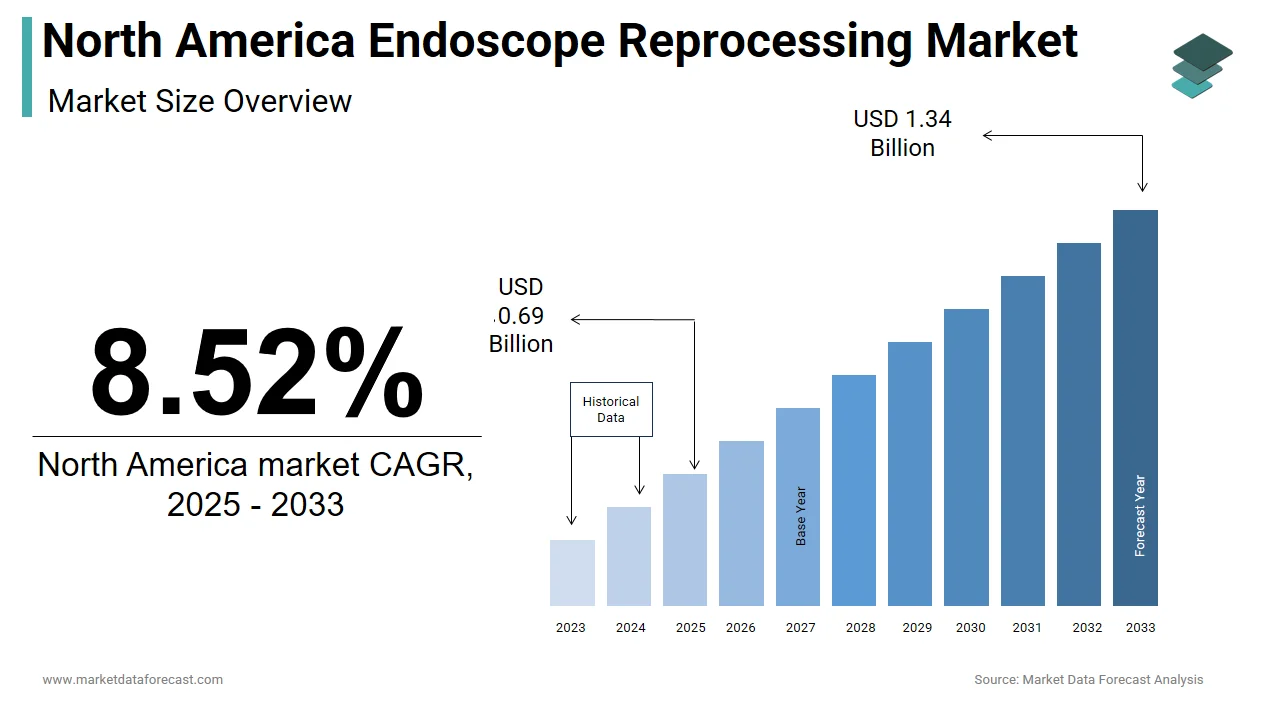
The size of the global North America endoscope reprocessing market was worth USD 0.64 billion in 2024. The global market is anticipated to grow at a CAGR of 8.52% from 2025 to 2033 and be worth USD 1.34 billion by 2033 from USD 0.69 billion in 2025.
MARKET DRIVERS
Increasing Endoscopic Procedures
The growing number of endoscopic procedures for various diseases across North America is one of the key factors driving the growth of the North American endoscope reprocessing market. The growing utilization of endoscopy for the screening, diagnosing, and treating of gastrointestinal diseases plays a pivotal role in propelling regional market growth. Recent years have witnessed nearly 40 percent of the American population suffering from various gastrointestinal ailments. Endoscopy, as a procedure, enables healthcare providers to examine the internal organs of individuals visually. It involves the insertion of a long tube through the mouth, uterus, or other relevant body openings, facilitating the detection of conditions such as gastroesophageal reflux disease, ulcers, cancer, inflammation, swelling, blockages, and other relevant issues prevalent among the population. The reprocessing of endoscope devices involves multiple steps, including precleaning, rinsing, drying, cleaning, rinsing, disinfection, and storage. These essential processes ensure the safe and effective reuse of endoscope devices.
Rising Geriatric Population
The growing geriatric population and the rising incidence of infections following endoscopic procedures across North America further fuel the growth rate of the North American endoscope reprocessing market. The aging population is more susceptible to various health issues, often requiring diagnostic procedures to detect underlying conditions. However, older individuals are also more vulnerable to infections within hospital settings due to weakened immune systems. Hospitals are increasingly adopting endoscope reprocessing methods performed by skilled professionals to mitigate the associated risks associated with infections. This proactive approach not only ensures patient safety but also contributes to the growth of the endoscope reprocessing market.
Advancements in Reprocessing Techniques
The growing number of advancements in reprocessing techniques contributes to regional market growth. Manufacturers and researchers are actively developing a diverse range of devices and techniques to enhance the cleaning process for endoscopic devices. This focus on innovation has led to the creation of specialized liquids and sterilization equipment that simplify and optimize the reprocessing procedure. The significant investments in research and development, the presence of key players dedicated to driving advancements, and the unwavering support of healthcare organizations promote endoscopy procedures in the North American region.
MARKET RESTRAINTS
High Costs of Endoscope Reprocessing and Procedures
The high cost associated with the reprocessing process for endoscopes and the escalating cost of endoscopic procedures primarily hamper the North American market growth. These cost-related aspects pose significant restraints on market expansion. Factors such as a shortage of skilled professionals proficient in endoscope reprocessing, limited availability of appropriate devices for cleaning, inadequate infrastructure facilities, and insufficient awareness about new techniques in the field further impede the regional market growth.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
Segments Covered |
By Product, End User, and Region. |
|
Various Analyses Covered |
Global, Regional and Country-Level Analysis, Segment-Level Analysis, Drivers, Restraints, Opportunities, Challenges; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Countries Covered |
United States, Canada, Mexico, and the Rest of North America. |
|
Market Leaders Profiled |
Advanced Sterilization Products (ASP) (US), Cantel Medical (US), Laboratories Anios (France), Olympus (Japan), Wassenburg Medical (The Netherlands), Custom Ultrasonics (US), STERIS (US), Steelco (Italy), Getinge (Sweden), ENDO-TECHNIK (Germany), BES Decon (UK), ARC Healthcare Solutions (Canada), and Metrex Research (US), among others. |
COUNTRY LEVEL ANALYSIS

North America held the major share of the worldwide market in 2024, and the domination of the region is expected to continue throughout the forecast period. The growing utilization of advanced reprocessing techniques for advanced medical devices used in diagnosis and treatment favors the market growth in the North American region.
The U.S. led the endoscope reprocessing market in North America in 2024; the lead of the U.S. market is estimated to continue during the forecast period. The government of the U.S. implemented guidelines for endoscope reprocessing procedures to minimize infection rates and enhance patient safety, which supports regional market growth. The rising awareness about the importance of early diagnosis and increasing healthcare expenditure among the U.S. population further boost the growth rate of the U.S. market. The presence of highly skilled professionals in endoscope reprocessing procedures, the introduction of novel products, the establishment of additional training centers focused on effective endoscopic reprocessing processes, and the rapid adoption of minimally invasive surgeries among the population drive the growth of the U.S. market.
Canada is estimated to account for a notable share of the North American market during the forecast period, owing to the rising number of laboratory centers and the adoption of endoscope devices by hospitals and specialized healthcare clinics. Hospitals in Canada make substantial investments in advanced infrastructure and implement advanced technology systems to reduce work stress on healthcare professionals. Furthermore, manufacturing centers in Canada prioritize the development of automatic endoscope reprocessing devices that require minimal human intervention.
KEY MARKET PLAYERS
Companies playing a dominant role in the North America Endoscope Reprocessing Market profiled in this report are Advanced Sterilization Products (ASP) (US), Cantel Medical (US), Laboratories Anios (France), Olympus (Japan), Wassenburg Medical (The Netherlands), Custom Ultrasonics (US), STERIS (US), Steelco (Italy), Getinge (Sweden), ENDO-TECHNIK (Germany), BES Decon (UK), ARC Healthcare Solutions (Canada), and Metrex Research (US), among others.
MARKET SEGMENTATION
This research report on the North America endoscope reprocessing market is segmented and sub-segmented into the following categories.
By Product
- High-level disinfectants And Test Strips
- Detergents And Wipes
- Automated Endoscope Reprocessors,
- Endoscope Drying
- Storage And Transport Systems
- Endoscope Tracking Systems
- Other Products
By End User
- Hospitals
- Ambulatory Surgery Centres
- Others
By Country
- United States
- Canada
- Mexico
- Rest of North America
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]