Latin America Vaccine Adjuvant Market Size, Share, Trends & Growth Forecast Report By Type (Aluminum Salts, Tensoactive Adjuvants, Adjuvant Emulsions, Bacteria Derived Adjuvants, Liposome Adjuvants, Carbohydrate Adjuvants), Route Of Administration, Mechanism Of Action and Country (Mexico, Brazil, Argentina, Chile and Rest of Latin America), Industry Analysis From 2025 to 2033
Latin America Vaccine Adjuvant Market Size
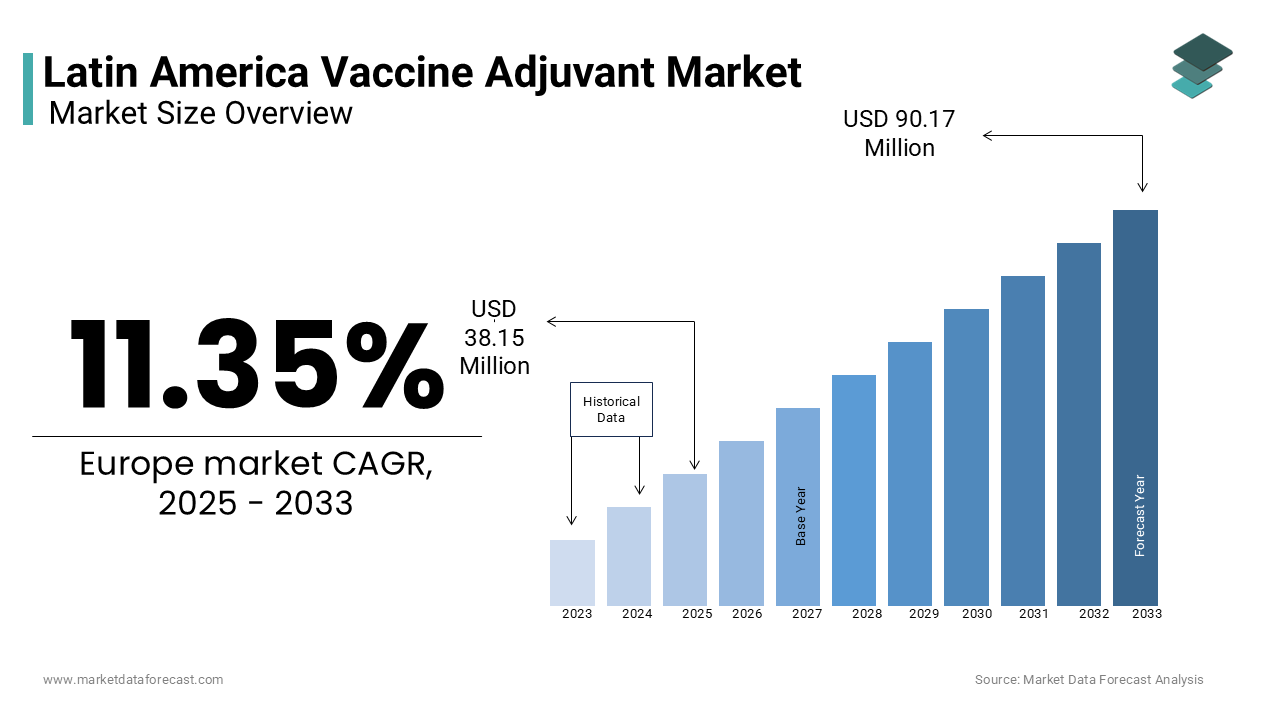
The Latin America Vaccine Adjuvant Market size was valued at USD 34.26 million in 2024. The vaccine adjuvant market in Latin America is expected to grow at a CAGR of 11.35% from 2025 to 2033 and be worth USD 90.17 million by 2033 from USD 38.15 million in 2025.
Vaccine adjuvants are substances added to vaccines to enhance the immune response and allow for greater efficacy, lower antigen doses, and longer-lasting immunity. These adjuvants play a crucial role in addressing public health challenges, particularly in regions like Latin America, where infectious diseases such as influenza, dengue, and Zika virus are prevalent. According to the Pan American Health Organization, immunization programs in Latin America have significantly improved disease prevention, with vaccine coverage rates for measles exceeding 90% in several countries. However, emerging diseases and waning immunity have increased the demand for vaccines with enhanced efficacy, spurring the use of adjuvants such as aluminum salts, oil-in-water emulsions, and novel adjuvant systems.
MARKET DRIVERS
Rising Prevalence of Infectious Diseases
The growing prevalence of infectious diseases is a significant driver of the Latin America vaccine adjuvant market. According to the Pan American Health Organization, diseases like dengue, influenza, and Zika virus continue to pose serious public health threats in the region. In 2022, over 3 million dengue cases were reported across Latin America, highlighting the urgent need for effective vaccines. Vaccine adjuvants enhance immune responses, making them critical in addressing such widespread health concerns. Additionally, endemic diseases like chikungunya and yellow fever further emphasize the importance of vaccines with advanced adjuvants, driving investment and development in the regional market.
Government Immunization Programs and Policies
Strong government-backed immunization programs and policies significantly propel the Latin America vaccine adjuvant market. The World Health Organization highlights that regional initiatives, such as the Expanded Program on Immunization, have led to high vaccination coverage rates for diseases like measles and hepatitis B, with rates exceeding 90% in countries such as Brazil and Mexico. These programs often utilize vaccines containing adjuvants to boost efficacy and ensure long-lasting immunity. Furthermore, efforts to strengthen COVID-19 vaccination campaigns have accelerated the adoption of adjuvant technologies, with governments investing in research and local production. This focus on immunization infrastructure continues to drive the growth of the vaccine adjuvant market across Latin America.
MARKET RESTRAINTS
High Cost of Vaccine Development and Production
The high cost associated with developing and producing vaccines with advanced adjuvants is a major restraint in the Latin America vaccine adjuvant market. According to the World Health Organization, the development of a single vaccine can cost upwards of $500 million, with adjuvant research contributing significantly to these expenses. Many countries in Latin America, such as Bolivia and Honduras, face limited healthcare budgets, making it challenging to invest in advanced vaccine technologies. Additionally, the reliance on imported vaccines increases costs further, as most adjuvant manufacturing facilities are located in developed countries. This financial burden restricts access to advanced vaccines, slowing market growth.
Inadequate Infrastructure for Vaccine Manufacturing
The lack of sufficient infrastructure for vaccine manufacturing in Latin America is another key challenge for the vaccine adjuvant market. The Pan American Health Organization notes that only a few countries, such as Brazil and Mexico, have robust vaccine production capabilities, while smaller economies rely heavily on imports. This uneven distribution of manufacturing capacity results in supply chain disruptions and delays in vaccine availability. Furthermore, inadequate research facilities for developing adjuvant technologies hinder innovation and local production. Strengthening regional infrastructure is essential to overcome this limitation and meet the growing demand for adjuvant-enhanced vaccines in Latin America.
MARKET OPPORTUNITIES
Growing Focus on Regional Vaccine Manufacturing
The increasing emphasis on regional vaccine manufacturing presents a significant opportunity for the Latin America vaccine adjuvant market. The Pan American Health Organization highlights initiatives like the Regional Platform for Advancing the Manufacturing of Vaccines and Health Technologies to enhance local production capacity. Countries such as Brazil and Argentina are investing in advanced facilities to produce vaccines with adjuvants locally, reducing dependency on imports. This development not only lowers production costs but also improves vaccine accessibility across the region. Furthermore, regional production enhances preparedness for future pandemics, supporting long-term market growth and fostering technological innovation in vaccine adjuvant development.
Advancements in Novel Adjuvant Technologies
The development and adoption of novel adjuvant technologies offer immense growth potential for the Latin America vaccine adjuvant market. According to the World Health Organization, next-generation adjuvants, such as saponin-based and synthetic emulsions, enhance vaccine efficacy against complex diseases like malaria and HIV. With increasing R&D investments, these advanced adjuvants are being integrated into pipeline vaccines for both infectious and non-communicable diseases. For instance, adjuvants that boost immune responses in elderly populations are gaining attention, particularly for diseases like influenza. These innovations align with the region's growing focus on improving vaccination outcomes, creating new avenues for the expansion of the vaccine adjuvant market.
MARKET CHALLENGES
Regulatory Hurdles and Fragmented Approval Processes
The complex and fragmented regulatory landscape in Latin America poses a significant challenge to the vaccine adjuvant market. The World Health Organization emphasizes that differences in regulatory requirements across countries like Brazil, Mexico, and Argentina lead to delays in vaccine approvals. For instance, while Brazil has a well-established regulatory authority in ANVISA, other nations lack uniform standards, causing inconsistencies in quality assurance and market entry timelines. These hurdles increase development costs and slow down the adoption of innovative adjuvants, limiting their availability in the region.
Limited Access to Funding and Investments
The scarcity of funding and investment for vaccine and adjuvant development in Latin America is a persistent challenge. According to the Pan American Health Organization, many countries in the region allocate less than 5% of GDP to healthcare, constraining resources for vaccine research and infrastructure development. Smaller economies like Honduras and El Salvador struggle to attract private investments, relying heavily on international aid. This financial limitation hampers the ability of regional manufacturers to innovate and produce vaccines with advanced adjuvants.
REGIONAL ANALYSIS
Brazil is the largest market for vaccine adjuvants in Latin America owing to the robust manufacturing infrastructure of Brazil and high vaccination coverage. The Pan American Health Organization reports that Brazil has achieved over 90% immunization rates for diseases like measles and polio through its National Immunization Program. ANVISA, the country’s regulatory authority, facilitates vaccine development and local production, reducing reliance on imports. Brazil’s focus on combating diseases like dengue, yellow fever, and COVID-19 has driven investments in adjuvant technologies. With its extensive healthcare system and significant government funding, Brazil leads the region in developing and distributing vaccines with advanced adjuvants.
Mexico plays a critical role in the Latin America vaccine adjuvant market due to its strong healthcare policies and increasing vaccine research. The Mexican Ministry of Health emphasizes immunization programs to combat diseases like influenza and HPV, which utilize adjuvant-enhanced vaccines. According to the Pan American Health Organization, Mexico maintains high vaccination rates, with over 85% coverage for childhood diseases. The country’s strategic partnerships with global vaccine manufacturers have bolstered local production capabilities. Additionally, Mexico’s proactive response during the COVID-19 pandemic, including the development and distribution of adjuvant-containing vaccines, has highlighted its importance in the regional market.
Argentina is a key contributor to the vaccine adjuvant market, driven by its advanced biopharmaceutical sector and government-backed immunization programs. The Pan American Health Organization reports that Argentina’s vaccination coverage for diseases like hepatitis B and tetanus exceeds 80%. The government’s commitment to universal healthcare ensures widespread access to vaccines, increasing demand for adjuvants.
KEY MARKET PLAYERS
Companies playing a prominent role in the Latin American vaccine adjuvant market profiled in this report are MPV Technologies, Avanti Polar Lipids, Novavax Inc., Brenntag Biosector, SEPPIC, Agenus, Inc., Invivogen, SPI Pharma, Inc., CSL Limited, and OZ Biosciences.
MARKET SEGMENTATION
This research report on the Latin American vaccine adjuvant market is segmented and sub-segmented into the following categories.
By Type
- Aluminum Salts
- Tensoactive Adjuvants
- Adjuvant Emulsions
- Bacteria Derived Adjuvants
- Liposome Adjuvants
- Carbohydrate Adjuvants
By Route Of Administration
- Oral
- Intradermal
- Subcutaneous
- Intranasal
- Intramuscular
By Mechanism Of Action
- Immuno Stimulants
- Carriers
- Vehicle Adjuvants
By Country
- Mexico
- Brazil
- Argentina
- Chile
- Rest of Latin America
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 1600
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
