Europe Microbiology Testing Market Size, Share, Trends & Growth Forecast By Microbiology Application, Clinical Application, Product and Country (Germany, UK, France, Italy, Rest of Europe) – Industry Analysis From 2025 to 2033.
Europe Microbiology Testing Market Size
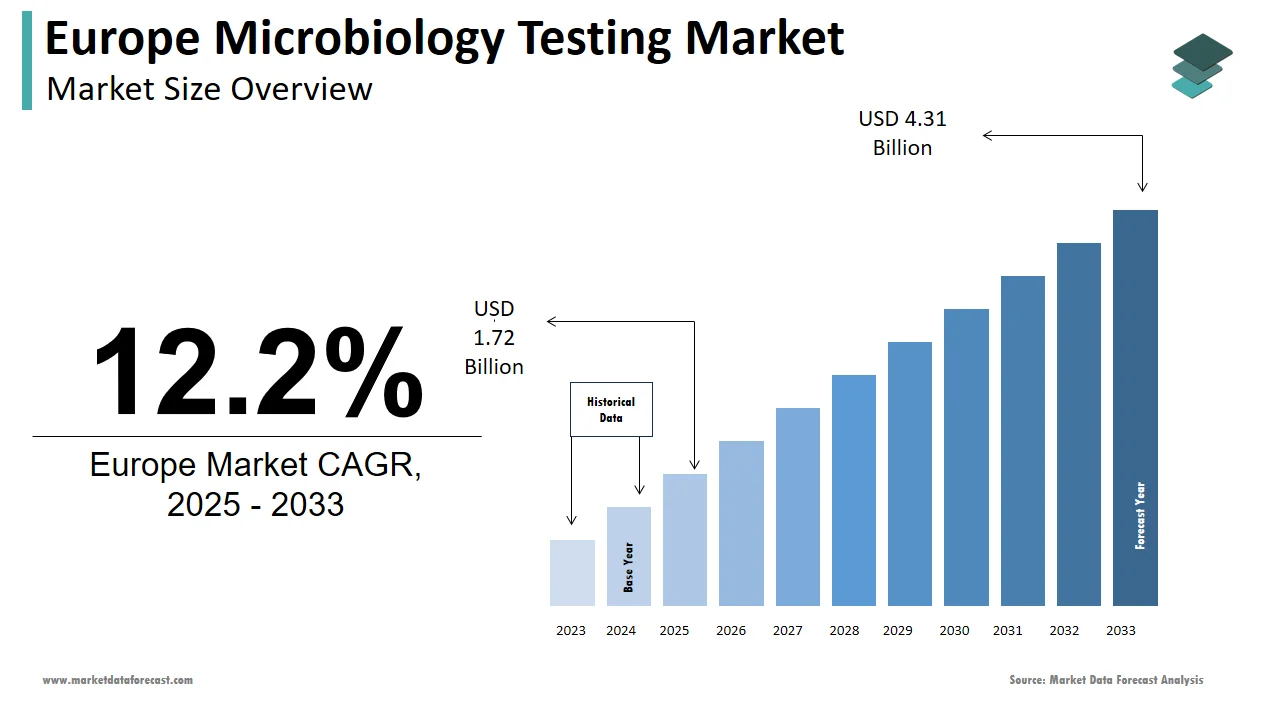
The size of the Europe microbiology testing market was valued at USD 1.53 billion in 2024. This market is expected to grow at a CAGR of 12.2% from 2025 to 2033 and be worth USD 4.31 billion by 2033 from USD 1.72 billion in 2025.
MARKET DRIVERS
Technological Advancements in Disease Diagnostics
Technological advancements in disease diagnostics across Europe are majorly driving the growth of the European market. Various advanced molecular diagnostic techniques are widely used to detect and effectively treat pathogen-borne diseases. Compared to traditional lab techniques, advanced molecular techniques offer various technical benefits such as high sample detection, specificity, accuracy, increased device sensitivity, and rapid results. Several techniques, such as real-time PCR, NGS, and PCR coupled with mass spectroscopy, are gaining high recognition for identifying and characterizing various pathogens and analysing multiple samples in a single runtime. These factors have increased technology adoption, boosting the European clinical microbiology market size. Many healthcare systems, such as hospitals, clinics, research institutes, and pharmaceutical companies, adopt these techniques to improve diagnostic services. Pathogen-specific diagnostic kits are gaining traction due to early diagnosis in less time by avoiding all the traditional tests for one pathogen. Microbiological testing is utilized for quality assurance for food and beverage products and cosmetic products.
Growing Prevalence of Pathogen-Based Diseases
The growing prevalence of pathogen-based diseases in Europe is further fuelling the growth rate of the European market. Countless pathogens are present around us, and we are exposed to numerous pathogens that are not harmful; only specific pathogens are harmful. Abundant new diseases have been marked in recent years due to pathogens requiring diagnosis and treatment. The increasing number of pathogens species makes end-users focus on innovative and technologically advanced molecular diagnostic techniques. Research laboratories and pharmaceutical companies focus on developing innovative treatment methods with diagnostic patterns. The European government is taking initiatives to develop advanced molecular diagnostic techniques. Specific pathogen diagnostic kits help reduce the time for diagnosis with rapid results, and multiple samples can be tested at a time.
Technological Advancements in Healthcare Systems
The increasing technology advancements in the healthcare systems in Europe will create opportunities in the market. Adopting advanced technology will move the healthcare systems toward digital transformation. The increasing government investments in the healthcare infrastructure are expected to create lucrative opportunities for the European clinical microbiology market. With the increasing investments in R&D by the manufacturers, microbiology testing will experience various possibilities by increasing the market revenue.
MARKET RESTRAINTS
Errors in Testing Stages
The clinical microbiology market is limited due to errors in the testing stages. Even one early-stage mistake leads to an overlap of the complete results. The manual data import should avoid overlapping results, such as incorrect identification. The procedures must be done in aseptic areas to avoid contamination, and the microbiologist must be careful with the surroundings. Another factor hampering the European clinical microbiology was the limited reimbursement policies for the testing procedures. The government considers only a few testing procedures under reimbursement, limiting the preference for microbiology testing among patients and healthcare professionals. National Standardization reimbursements need to include some regions affecting the regional market.
Barriers in Operational Procedures
The major challenge the European microbiology testing faced was the barriers situated during operational procedures. The samples collected by the labs need to be transported safely with specific temperature requirements and standardized sampling procedures for getting accurate and correct diagnostic results of patients. Patient samples are taken from saliva, semen, tissue, blood, and urine; these have limited the use of automated instruments that directly diagnose common pathogens such as Plasmodium, Staphylococcus, and E. coli.
COUNTRY LEVEL ANALYSIS
The European region is ranked second in global microbiology testing due to its advanced healthcare systems. Germany held the dominant share in the European market revenue due to its technological advancements and increasing government investments in developing healthcare infrastructure. Many small clinical laboratories lack the required knowledge of molecular technologies that can use simple and modified, less expensive labor-intensive polymerase chain reaction methods (PCR), accelerating the market growth in this region. Microbiology testing was necessary to determine the correct treatment method and effective measures, which played a crucial role in the eradication of the recent pandemic.
The UK is one of the most modernized countries; it adopts technological advancements in all fields, including the healthcare system. People are becoming more prone to infectious diseases, requiring an accurate diagnosis for the correct treatment process, raising the market share. France and Italy are expected to have a decent growth rate in the upcoming years, as infections are increasing and microbiology testing is crucial. To ensure the quality of the products in food and beverages, pharmaceuticals, and skin care, microbiology testing was necessary to avoid the harmful effects on humans. Spain is also considered to grow by influencing the European microbiology testing market value in the forecast period.
KEY MARKET PLAYERS
Danaher Corporation, Becton, Dickinson, and Company, Cepheid Abbott Laboratories Inc., Bio-Rad Laboratories Inc., F. Hoffman-La Roche Ltd. (Switzerland), Alere Inc., Bruker Corporation, and Hologic, Inc. are some of the leading companies in the European microbiology testing/clinical microbiology market.
MARKET SEGMENTATION
This Europe microbiology testing market research report is segmented and sub-segmented into the following categories.
By Microbiology Application
- Pharmaceutical
- Clinical
- Manufacturing
- Energy
By Clinical Application
- Respiratory Diseases
- STD
- UTI
By Product
- instruments
- Analyzers
- Consumables
By Country
- UK
- France
- Spain
- Germany
- Italy
- Russia
- Sweden
- Denmark
- Switzerland
- Netherlands
- Turkey
- Czech Republic
- Rest of Europe
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]