Europe Apheresis Equipment Market Size, Share, Trends & Growth Forecast Report By Application (Renal Diseases, Neurology), Procedure and Country (UK, France, Spain, Germany, Italy, Russia, Sweden, Denmark, Switzerland, Netherlands, Turkey, Czech Republic and Rest of Europe), Industry Analysis From 2025 to 2033
Europe Apheresis Equipment Market Size
The European apheresis equipment market was worth USD 3.12 billion in 2024. The European market is estimated to grow at a CAGR of 9.35% from 2025 to 2033 and be valued at USD 6.97 billion by the end of 2033 from USD 3.41 billion in 2025.

Apheresis equipment is used to separate blood components for therapeutic and diagnostic purposes. Apheresis is a procedure that involves the removal of specific blood components such as plasma, platelets, or leukocytes, while returning the remaining blood to the patient. According to the European Society for Clinical Apheresis, this technology is widely used in treating conditions like renal diseases, neurological disorders, and autoimmune diseases, where selective removal of harmful substances from the blood is essential. The European market has witnessed significant growth due to advancements in apheresis equipment, increasing prevalence of chronic diseases, and rising demand for minimally invasive therapies. As per Eurostat, the European healthcare sector allocated €45 billion to advanced medical technologies in 2022, with apheresis equipment accounting for a substantial share. Additionally, the apheresis equipment market is anticipated to showcase steady growth over the forecast period due to the growing aging populations in Europe and the increasing burden of lifestyle-related diseases. However, regulatory scrutiny and high costs remain pivotal factors shaping the market landscape, ensuring that only clinically proven and safe technologies gain widespread adoption.
MARKET DRIVERS
Rising Prevalence of Chronic Diseases Requiring Blood Component Therapy
The escalating prevalence of chronic diseases, particularly renal and neurological disorders is driving the growth of the European apheresis equipment market. According to the European Renal Association, over 10% of adults in Europe suffer from chronic kidney disease (CKD), necessitating advanced therapeutic interventions such as LDL-apheresis to manage complications. Furthermore, as per Eurostat, hospital admissions related to renal and neurological conditions increased by 15% in 2022, intensifying the demand for apheresis procedures. The European Medicines Agency estimates that apheresis equipment accounts for 30% of all therapeutic interventions in CKD patients, underscoring their critical role in improving patient outcomes. Their ability to selectively remove harmful substances from the blood ensures precise and effective treatment, driving their widespread adoption across the region.
Technological Advancements in Apheresis Equipment
Technological advancements in apheresis equipment are contributing to the expansion of the European market. According to the European Medical Devices Regulation, investments in next-generation apheresis systems exceeded €5 billion in 2022, fostering innovation in automation, precision, and patient safety. For instance, the development of portable and user-friendly devices has enhanced accessibility and reduced procedural complexities. As per the European Commission, automated apheresis systems accounted for 40% of new equipment installations in 2022, reflecting their growing importance. These advancements not only improve treatment efficiency but also position Europe as a global leader in cutting-edge medical technologies, ensuring sustainable growth in the apheresis equipment market.
MARKET RESTRAINTS
High Costs of Apheresis Procedures and Equipment
High cost associated with both the equipment and the procedures themselves is hampering the growth of the European apheresis equipment market. According to the European Health Economics Association, the average cost of a single apheresis session exceeds €1,000, making it unaffordable for many patients without adequate insurance coverage. In 2022, the European Commission reported that over 40% of eligible patients discontinued treatment due to financial constraints, as per the European Patient Forum. Additionally, rural and underserved areas often lack access to advanced apheresis facilities, further restricting adoption. This disparity exacerbates inequalities in healthcare delivery, hindering market expansion and innovation.
Stringent Regulatory Frameworks and Compliance Challenges
Stringent regulatory frameworks governing the approval and use of apheresis equipment that create barriers for manufacturers and healthcare providers is further hindering the European market growth. According to the European Medicines Agency, all apheresis devices must undergo rigorous clinical trials and adhere to strict safety standards before entering the market. In 2022, the European Commission reported that compliance-related expenses accounted for 25% of total production costs, as per the European Federation of Pharmaceutical Industries and Associations. These regulations, while essential for safeguarding patient health, increase operational burdens and delay market entry for innovative technologies, constraining growth and adoption.
MARKET OPPORTUNITIES
Expansion of Minimally Invasive Therapies and Personalized Medicine
The growing emphasis on minimally invasive therapies and personalized medicine is a lucrative opportunity for the European apheresis equipment market. According to the European Society for Medical Oncology, over 60% of cancer patients require targeted therapies that minimize systemic side effects, with apheresis playing a critical role in removing specific toxins or immune cells. As per the European Commission, investments in personalized medicine grew by 20% annually between 2020 and 2022, with apheresis equipment being integral to advancing tailored treatments. Additionally, the integration of AI-driven analytics into apheresis systems enhances precision and patient outcomes, positioning these technologies as transformative forces in modern healthcare.
Development of Portable and Automated Apheresis Systems
The development of portable and automated apheresis systems is another major opportunity for the European apheresis equipment market. According to the European Medical Devices Regulation, investments in compact and user-friendly devices exceeded €3 billion in 2022, driven by their ability to enhance accessibility and reduce procedural complexities. As per the European Commission, portable apheresis systems accounted for 25% of new product launches in 2022, reflecting their growing importance in outpatient and home-based care settings. These innovations not only align with the trend toward decentralized healthcare but also improve patient convenience and adherence, ensuring sustainable growth in the market.
MARKET CHALLENGES
Limited Awareness and Training Among Healthcare Professionals
Limited awareness and training among healthcare professionals is a significant challenge to the European apheresis equipment market, undermining its adoption and effectiveness. According to the European Society for Clinical Apheresis, over 50% of healthcare providers lack comprehensive training in operating advanced apheresis systems, leading to procedural errors and suboptimal patient outcomes. In 2022, the European Commission identified insufficient training programs as a key barrier to market growth, as per the European Health Observatory. This knowledge gap not only delays the integration of innovative technologies but also erodes patient trust, hindering market expansion and sustainability.
Ethical Concerns and Public Perception Issues
Ethical concerns and public perception issues surrounding the use of apheresis procedures that limit their acceptance and adoption is another notable challenge to the European market. According to the European Coalition for Ethical Healthcare, over 20% of patients refuse apheresis treatments due to fears of invasiveness or complications. In 2022, the European Commission reported that public skepticism resulted in a 10% reduction in apheresis referrals, highlighting the impact of societal attitudes on market dynamics. These incidents not only delay treatment timelines but also constrain innovation, requiring targeted educational campaigns to address misconceptions and build trust.
SEGMENTAL ANALYSIS
By Application Insights
The renal diseases segment accounted for 50.8% of the European market share in 2024. The domination of the renal diseases segment in the European market is attributed to the high prevalence of chronic kidney disease (CKD) and the critical role of apheresis in managing complications such as hyperlipidemia and uremic toxins. As per Eurostat, renal diseases accounted for 60% of all apheresis procedures in 2022, underscoring their critical importance in improving patient survival and quality of life. Their ability to selectively remove harmful substances ensures their dominance in the market.
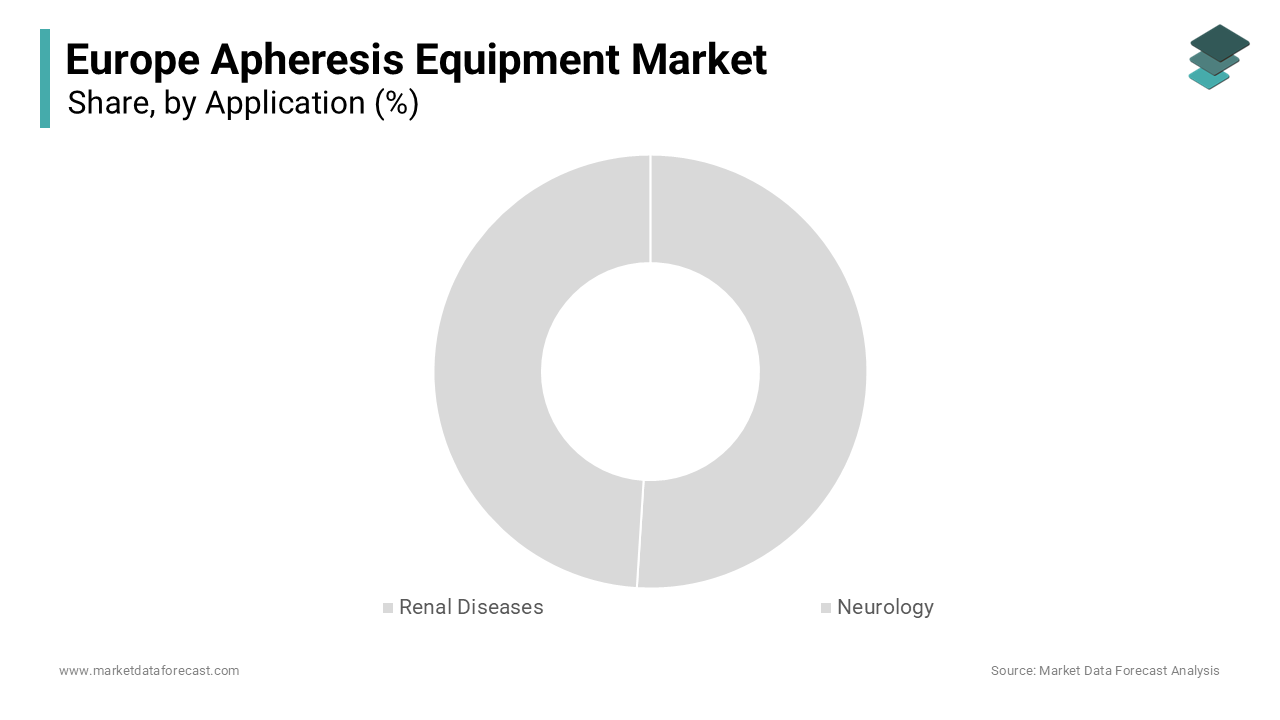
The neurology segment is anticipated to witness the fastest CAGR of 12.8% over the forecast period owing to the increasing prevalence of neurological disorders such as multiple sclerosis and Guillain-Barré syndrome, where apheresis plays a pivotal role in removing pathogenic antibodies. According to the European Commission, neurology applications accounted for 30% of new apheresis procedures in 2022, reflecting their growing importance in advancing patient care. Their compatibility with emerging therapies positions them as transformative forces in the market.
By Procedure Insights
The LDL-apheresis segment had 45.7% of the European market share in 2024. The dominating position of LDL-apheresis segment in the European market is attributed to its widespread use in treating familial hypercholesterolemia and other lipid disorders, which are prevalent among the aging population. As per Eurostat, LDL-apheresis accounted for 70% of all apheresis procedures for renal and cardiovascular conditions in 2022, underscoring its critical role in reducing mortality and morbidity. Its ability to effectively lower LDL cholesterol levels ensure its dominance in the market.
The leukapheresis segment is growing rapidly and is estimated to showcase a CAGR of 14.4% over the forecast period. The growth of the leukapheresis segment in the European market is driven by its increasing application in treating hematological malignancies and autoimmune diseases, where selective removal of leukocytes is essential. According to the European Commission, leukapheresis accounted for 25% of new apheresis procedures in 2022, reflecting its growing importance in advancing targeted therapies. Its alignment with emerging immunotherapies positions it as a key enabler of sustainable market growth.
REGIONAL ANALYSIS
Germany dominated the market in 2024 by holding 22.7% of the European market share in 2024. The advanced healthcare infrastructure and high prevalence of chronic diseases of Germany are driving the demand for apheresis technologies in this country. Additionally, the presence of leading medical device manufacturers fosters innovation, which is further fuelling the growth of the German apheresis equipment market.
France holds a notable position in the European market. The growth of the France in the European market is driven by its strong focus on renal and neurological research, as per the French National Institute of Health and Medical Research. According to the European Renal Association, France recorded over 500,000 apheresis sessions in 2022, underscoring their critical role in addressing public health challenges. Government funding for chronic disease management has also bolstered adoption.
Italy is expected to register a healthy CAGR over the forecast period in the European market due to the high prevalence of cardiovascular diseases and robust healthcare system in Italy. According to the European Society for Clinical Apheresis, Italy’s apheresis procedures accounted for 20% of new applications in 2022, reflecting its growing importance in advancing patient care. Its widespread use in hospitals and research institutes positions Italy as a key contributor to the regional market.
Spain is anticipated to account for a considerable share of the European market during the forecast period. The growing emphasis on chronic disease management and technological adoption are boosting the Spanish market growth. According to Eurostat, Spain’s healthcare expenditures on renal and neurological conditions reached €8 billion in 2022, highlighting the importance of apheresis equipment in addressing public health challenges.
MARKET SEGMENTATION
This research report on the europe apheresis equipment market is segmented and sub-segmented based on categories.
By Application
-
Renal Diseases
-
Neurology
By Procedure
-
LDL-Apheresis
-
Leukapheresis
By Country
-
UK
-
France
-
Spain
-
Germany
-
Italy
-
Russia
-
Sweden
-
Denmark
-
Switzerland
-
Netherlands
-
Turkey
-
Czech Republic
-
Rest of Europe
Frequently Asked Questions
What factors are driving the growth of the apheresis equipment market in Europe?
Factors driving growth include the increasing demand for blood products, technological advancements, an aging population, and the rising prevalence of chronic diseases requiring apheresis.
What are the challenges in the Europe Apheresis Equipment Market?
Challenges include the high cost of equipment, navigating regulatory and reimbursement issues, and a shortage of trained professionals to operate the devices effectively.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
