Global Clinical Trials Market Size, Share, Trends, COVID-19 Impact & Growth Forecast Report By Phase (Phase I Trials, Phase II Trials and Phase III Trials), Design (Interventional Trials, Observational Trials and Expanded Access Trials), Indication & Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa) - Industry Analysis, 2024 to 2032
Global Clinical Trials Market Size (2024 to 2032)
As per our report, the global clinical trials market size will grow to USD 42.87 Billion by 2032 from USD 23.06 Billion in 2024 and is expected to witness a CAGR of 7.83% during the forecast period from 2024 to 2032.
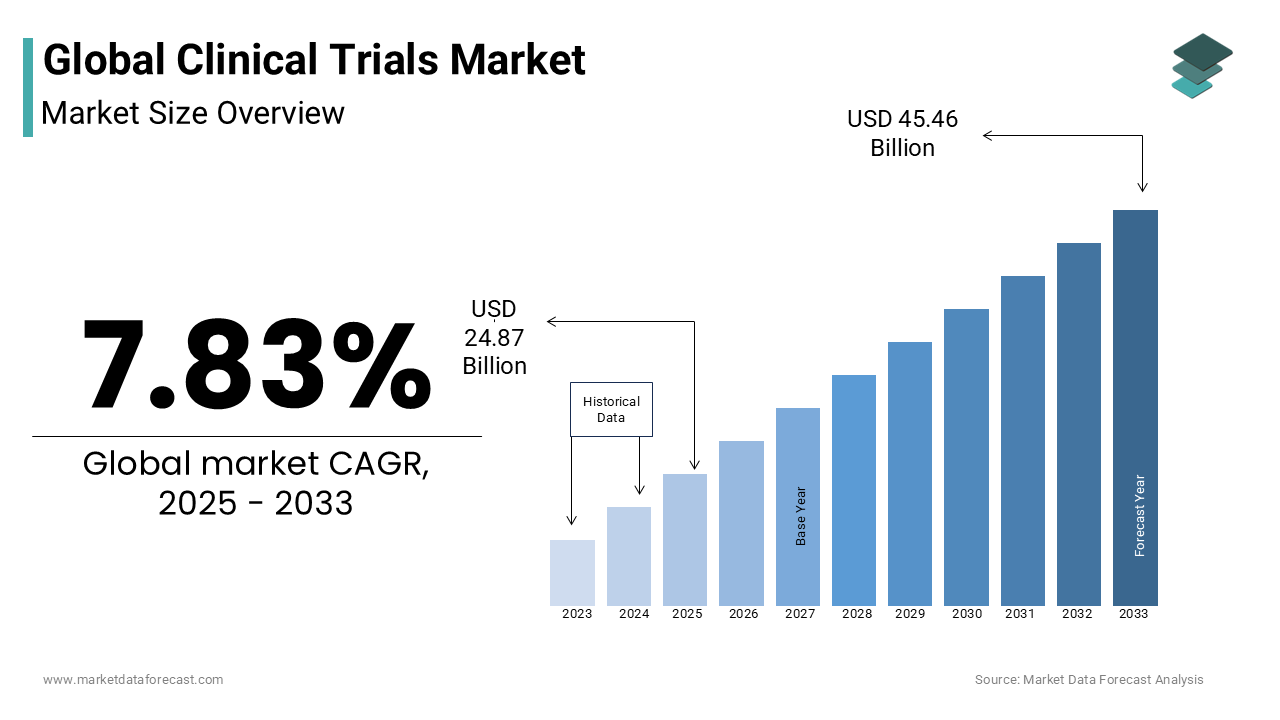
Current Scenario of the Global Clinical Trials Market
Clinical trials are a type of research that studies new tests and treatments by evaluating their effects on human health outcomes. Most people volunteer to participate in clinical trials to test medical interventions, including drugs, cells, and other biological products, surgical procedures, radiological procedures, devices, and preventive care. Clinical trials consist of four major phases, which are carefully designed, reviewed, and completed and need to be approved before the start of the procedure. The global clinical trials market accounted for significant growth and is anticipated to have notable growth during the forecast period. Companies' increased focus on developing novel treatments for chronic diseases and the escalating demand for outsourcing R&D activities are the primary factors driving market growth.
MARKET DRIVERS
The growing patient population of chronic diseases, increasing advances in biotechnology and personalized medicine, rising demand for innovative treatment options and the expansion of contract research outsourcing are majorly driving the growth of the clinical trials market.
Technological advancements, increasing demand for innovative solutions in the healthcare industry, and boosted alliances between the pharma-biotech companies and clinical research organizations are primarily driving the growth of the global clinical trials market. Ongoing research to ensure better treatment procedures for the patient's well-being is a significant factor that is opening growth opportunities for the global clinical trials market. The rise in the scale of pharmaceutical and biopharmaceutical companies is to surge growth opportunities for the global clinical trials market. The growing patient flow in hospitals and clinics is resulting in an increasing demand for effective treatment options, which is eventually contributing to an increasing number of clinical trial activities and propelling the global market growth.
The growing prevalence of personalized medicine in urban areas is fueling the growth of the clinical trials market. Accepting the latest technological developments and launching innovative products are spurring the market's growth rate. Furthermore, rising urbanization across the world is prompting the growth rate of the Global Clinical Trials Market to an extent.
The growing number of clinical trials owing to the increased investments by the major market players in the research activities to meet the requirements of the medications is expected to augment the market growth. The increasing rate of clinical trials every year is due to the rising demand for innovative and effective medications, escalating the market's growth opportunities. The presence of a strong pipeline of drugs in the clinical trials and the growing government support initiatives will enhance the market revenue in the coming years. According to the data provided by the WHO International Clinical Trials Registry Platform (ICTRP), in February 2023, around 19% of the drugs were in Phase I, 35% were in Phase II, 28% were in Phase III, and the remaining 18% of trails are yet to be started.
MARKET RESTRAINTS
The rising competition among pharmaceutical and biopharmaceutical companies demands unique and innovative novel treatment methods and drugs, which is a significant challenge for manufacturers in expanding their market revenue.
The need for continuous adoption of advanced technologies in the healthcare system and additional investments to change the reach of innovations to meet the disease prevalence is estimated to hamper the market growth due to limited successful results. The higher chances of clinical trials failing in the second phase due to drug safety and efficacy will negatively influence clinical trial market growth due to limited investments. The presence of stringent regulations for the approval of the phase and proceeding to the next phase regarding drug safety and efficacy and the high costs of drug design and development will hinder the market growth opportunities. The development of innovative drug delivery systems poses different challenges due to various requirements in chemistry, manufacturing, and the approvals of the applications, which all act as hurdles in the market expansion. The shortage of skilled professionals in clinical trials is another primary factor restraining the market expansion. Skilled professionals are in high demand in the clinical trial industries, biotech firms, and pharmaceuticals, which negatively influence the market growth rate. The availability of limited human subjects for clinical trials is one of the major factors restraining the global market growth rate. The side effects and risks associated with clinical trials limit the adoption among the people, restricting the availability of human subjects and leading to restricted market growth.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 to 2032 |
|
Base Year |
2023 |
|
Forecast Period |
2024 to 2032 |
|
Segments Covered |
By Phase, Design, Indication, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, Drivers, Challenges, Opportunities, Restraints, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Market Players |
Chiltern, Omnicare, PPD, Parexel, Kendle International Inc., Quintiles, ICON Plc, and Charles River. |
SEGMENTAL ANALYSIS
Global Clinical Trials Market Analysis By Phase
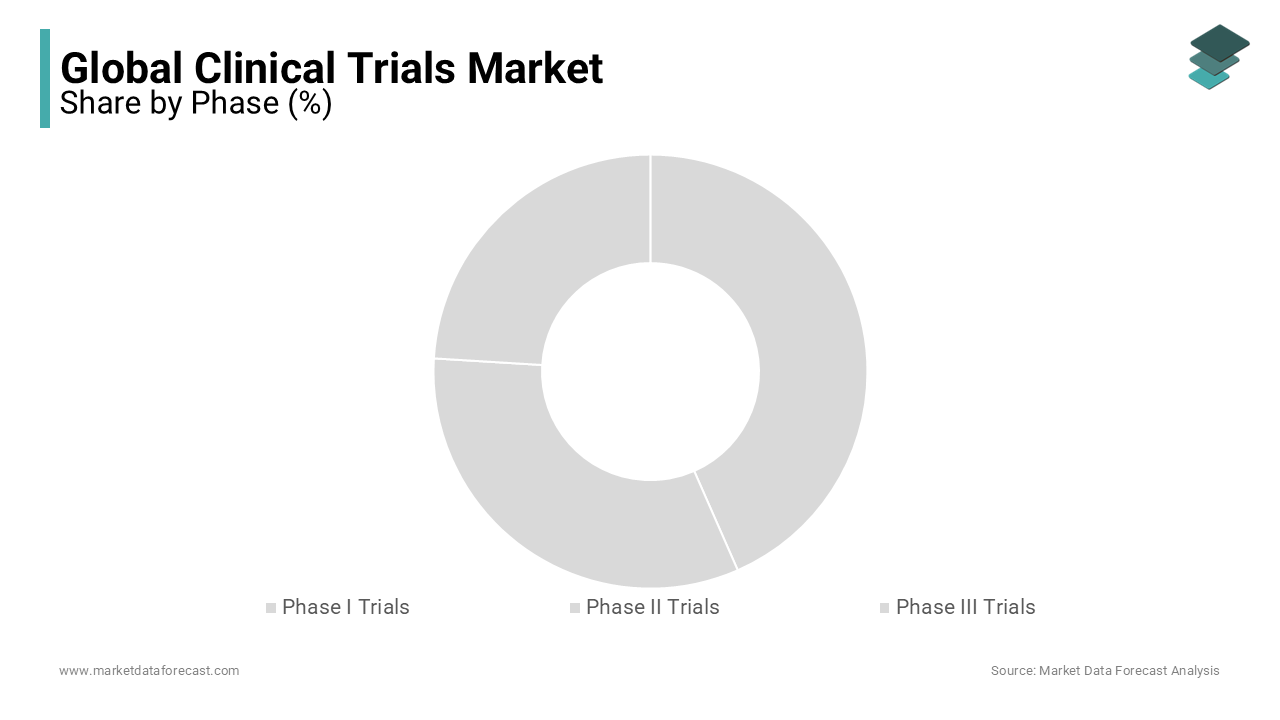
Based on the phase, the Phase III trials segment accounts for a market share of 53% in the global clinical trials market. The segment growth is majorly driven due to the growing per capita income, particularly in developed and developing countries. The growing number of patients with rare diseases is further propelling the growth of the phase III trials segment in the global market.
Phase II trials follow Phase III trials in much of the global market share and accounted for a market share of 18.9% in 2023. Phase II plays a vital role in cancer-related research. Rising disposable income in urban areas and the growing prevalence of quality treatment procedures are escalating the growth of this segment.
Global Clinical Trials Market Analysis By Design
The segment of interventional trails has had a tremendous growth rate over the past few years and occupied a share of 45% in terms of revenue in 2023. Increasing focus on developing new procedures for different diseases is leveraging the demand of the market. Increasing the scale of laboratories with the latest equipment and developing quality drugs is broadening the need for the clinical trials market. Furthermore, the concentration on developing new methods to treat various diseases and increasing demand for intervention by key players for clinical trials research.
The expanded access trials segment is next to Interventional trials in leading the market shares and growing at the highest CAGR value of 5.2% during the period owing to the rising awareness of the availability of different technologies.
Global Clinical Trials Market Analysis By Indication
The cancer segment dominated the global clinical trials market with the most significant share and is expected to dominate during the forecast period. The rising prevalence of cancer cases worldwide is the major factor contributing to the segment growth rate, as cancer is the leading cause of death across the globe. According to various data sources, more than 2000 cancer clinical trials have started, and many others are still in progress in 2023. These include various therapies such as cell and gene therapies, antibody-drug conjugates, multi-specific antibodies, and others. The increasingly effective cancer treatment therapies and a growing number of drug approvals for cancer are boosting the segment revenue expansion.
The cardiovascular system segment is expected to grow notably during the forecast period. The increased prevalence of cardiovascular diseases owing to the rise in the number of senior people with chronic diseases and cardiovascular diseases as one of the leading causes of death are a few primary factors driving the segment with growth opportunities.
REGIONAL ANALYSIS
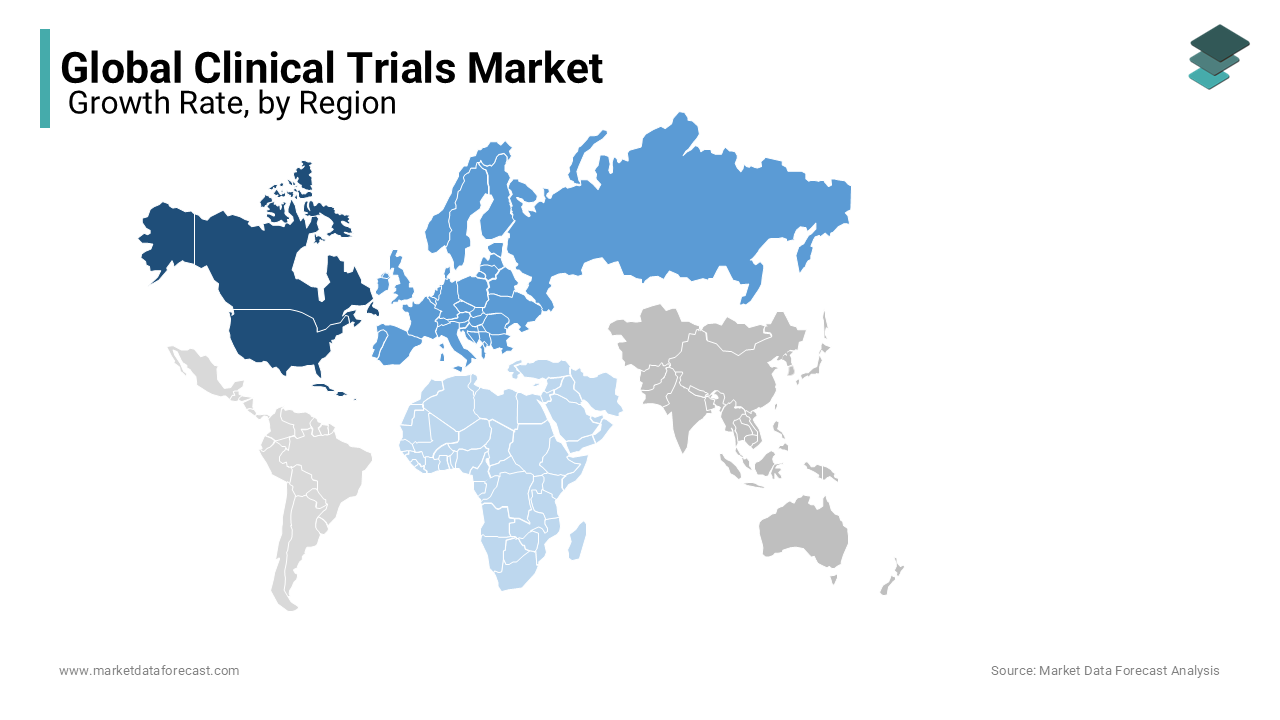
Geographically, the North American market is the biggest in terms of market share across the globe clinical trials market, followed by Europe. Ample availability of funds to outsource Clinical Trials serves as a significant growth driver for North America. The factors responsible for the growth of the market in this region are due to the appearance of eminent pharmaceutical production industries and integration with growing funds for the development of new vaccines. The United States is to start clinical trials using an artificially synthesized antibody, generally called gimsilumab. In this pandemic situation, scientists at Temple University Hospital have already used this therapy to treat high-risk patients. Gimsilumab is very effective in treating lung injury and other respiratory distress syndromes. So, the researchers strongly believe this therapy will work on the patients, and these clinical trials will help evaluate promising treatment procedures for COVID-19 patients.
The European regional market has occupied second place with the largest revenue and will likely produce profitable growth throughout the period. Germany is the leading nation in terms of factors like support from the government for research and development for new treatment procedures and upcoming infectious diseases and chronic diseases. The spread of analytics and awareness of its potential is increasing at a rapid rate in Europe.
The Asia-Pacific market is estimated to grow at the highest CAGR during the forecast period, which is significant due to initiatives from the government and contributions from academic laboratories, which helped augment growth. Rising demand for people suffering from cardiovascular diseases, cancer, and obesity in this region creates scope for the market to grow. In countries like China and Japan, the market growth is due to the increasing elderly population and common age-related health issues. Moreover, growing funds from the government and private organizations to supply medicines at an affordable price boost the growth rate of the market in this region.
The Latin American region is observed to have a promising growth rate during the forecast period. Factors like increasing safety concerns about people infected by COVID-19 have caused the market to have a substantial growth rate from 2023. 90% of the Brazilian population is suffering from various diseases, and it is an advantage for the laboratories to perform clinical trials and develop a vaccine. These are some factors soaring up market growth.
The Middle East & Africa market is estimated to have inclined growth during the forecast period and produce profitable growth in terms of revenue. In countries like the UAE and Saudi Arabia, growth is propelled by growing financial funds from the government. Moreover, a lack of scientific knowledge and a very small number of clinical trial centers, particularly in the African regions, hamper the market growth of the LATAM region.
KEY PLAYERS IN THE GLOBAL CLINICAL TRIALS MARKET
Some of the noteworthy companies dominating the global clinical trials market profiled in this report are Chiltern, Omnicare, PPD, Parexel, Kendle International Inc., Quintiles, ICON Plc, and Charles River.
RECENT DEVELOPMENTS IN THE CLINICAL TRIALS MARKET
- In August 2023, Parexel and Partex entered a strategic partnership to utilize Artificial Intelligence (AI) driven solutions in drug discovery and development for biopharmaceutical clients worldwide. The collaboration aimed to reduce risks associated with the assets in their respective portfolios.
- In August 2023, Novo Nordisk announced the acquisition of Inversago Pharma. This acquisition was part of Novo Nordisk’s strategic efforts to develop new therapies targeting individuals with obesity, diabetes, and other significant metabolic diseases.
- In December 2023, Thermo Fisher Scientific Inc. introduced CorEvidence, a cloud-based optimization of pharmacovigilance case processing and safety data management processes.
- In April 2022, Charles River Laboratories International Inc. disclosed its acquisition of Explora BioLabs Holdings, Inc., a leading provider of contract vivarium research services.
- The National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH) of the United States, started an early-stage clinical trial in July 2022 to investigate an investigational vaccine to stave off Nipah virus infection.
- In May 2022, the International AIDS Vaccine Initiative and Moderna Inc. started a Phase I clinical trial of an mRNA vaccine antigen in South Africa.
DETAILED SEGMENTATION OF THE GLOBAL CLINICAL TRIALS MARKET INCLUDED IN THIS REPORT
This research report on the global clinical trials market has been segmented based on phase, design, indication, and region.
By Phase
- Phase I Trials
- Phase II Trials
- Phase III Trials
By Design
- Interventional Trials
- Observational Trials
- Expanded Access Trials
By Indication
- Autoimmune
- Blood disorders
- Cancer
- Circulatory
- CNS
- Congenital
- CVS
- Dermatology
- Ear
- Gastrointestinal
- Genitourinary
- Infections
- Mental disorders
- Metabolic
- Musculoskeletal
- Nose
- Ophthalmology
By Region
- North America
- U.S
- Canada
- Europe
- U.K
- Germany
- France
- Spain
- Italy
- Asia Pacific
- India
- Japan
- China
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Middle East & Africa
- Middle East
- Africa
Frequently Asked Questions
What is the expected market size of the clinical trials market worldwide?
The global clinical trials market size is expected to reach USD 42.57 billion by 2032.
what is the compound annual growth rate (%) of the global clinical trials market during the forecast period?
The clinical trials market size is forecasted to grow at a CAGR of 7.83% from 2024 to 2032.
Which region holds the biggest market share of the clinical trials?
North America region holds the biggest share of the global clinical trials market.
Who are the leading players in the clinical trials market?
Chiltern, Omnicare, PPD, Parexel, Kendle International Inc, Quintiles, ICON Plc, and Charles River are the companies that plays a crucial role in the global clinical trials market.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
