Global Clinical Trial Management System Market Size, Share, Trends & Growth Forecast Report By Delivery Mode, Component, Type of System, End-User and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), Industry Analysis From 2025 To 2033.
Global Clinical Trial Management System Market Size
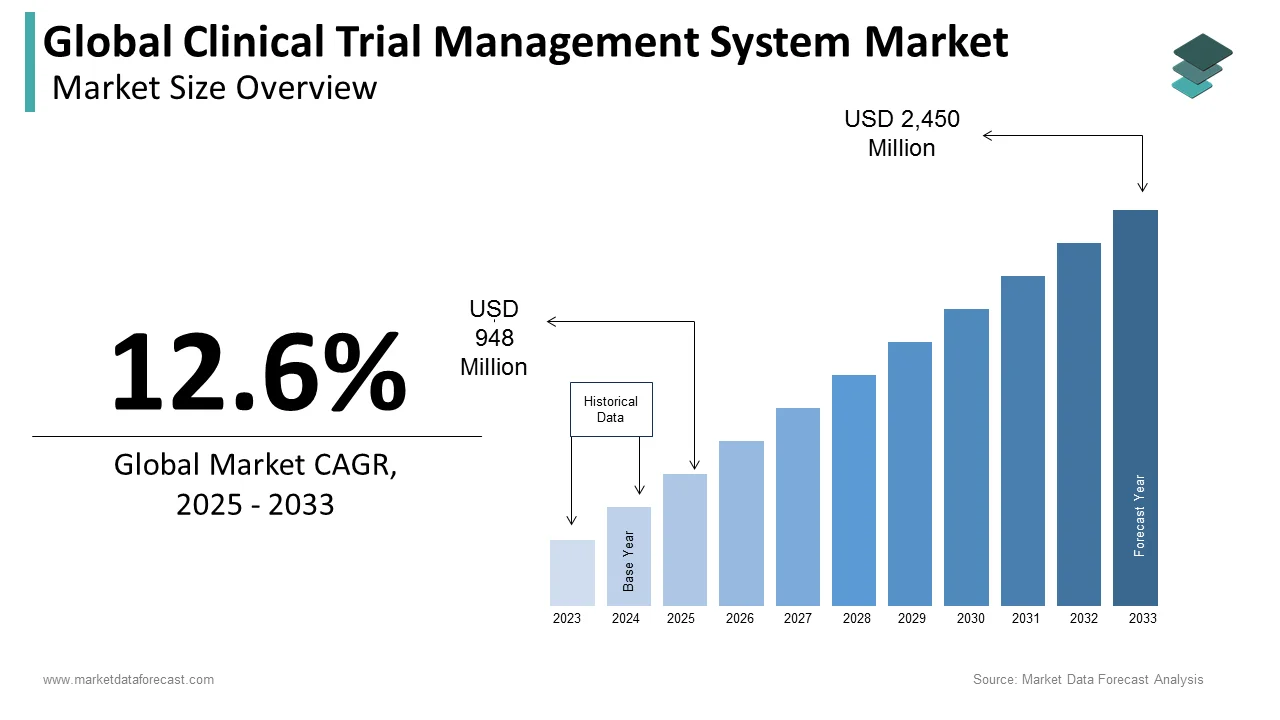
The size of the global clinical trial management system market was worth USD 842 million in 2024. The global market is anticipated to grow at a CAGR of 12.6% from 2024 to 2033 and be worth USD 2,450 million by 2033 from USD 948 million in 2025.
MARKET DRIVERS
The growing outsourcing of clinical trials by pharmaceutical companies and rising R&D activities majorly driving drive the CTMS market growth.
Outsourcing of clinical trials is very common in the pharmaceutical industry and pharmaceutical companies prefer this option to focus on their core competencies. Outsourcing clinical trials to CROs and other specialized service providers also helps pharmaceutical companies to leverage their expertise and resources efficiently. Pharmaceutical companies invest significant amounts to conduct R&D for novel drugs and therapies. In the process, the pharmaceutical companies go through several phases of clinical trials and effective management of clinical trials is crucial for the successful development of the drug. CTMS solutions help significantly and support the R&D process by offering required tools and functionalities to support the planning, execution, and monitoring of clinical trials. The demand for efficient management and organization of clinical trial operations has increased significantly in recent years. The trend is expected to continue in the coming years and boost the adoption of CTMS solutions by pharmaceutical companies and contract research organizations.
The growing patient population of chronic diseases, increasing demand for effective treatment procedures, rising investments in healthcare centers by governmental organizations and the availability of advanced CTMS solution technologies contribute to the CTMS market growth. The growing investments to conduct clinical trials in the life sciences industry, the increasing population worldwide, the shelf life of blockbuster drugs, and new system implementation in various industries favor the clinical trials management system market growth. The growing emphasis on dose accuracy, new administration routes and rising demand for target therapy for a particular disease drive the CTMS market growth. Furthermore, the growing number of R&D institutes in biotechnology with the latest equipment, the emergence of the latest technologies and the launch of innovative products with this system is surging growth opportunities for the clinical trial management system market.
MARKET RESTRAINTS
However, frequent updates in the software and the high costs associated with installing the systems are expected to limit the global clinical trial management system market growth. In addition, the lack of proper awareness about the benefits associated with CTMS is further estimated to inhibit market growth to a small extent.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
Segments Covered |
By Delivery Mode, Component, Type of System, End-User, and Region. |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis; DROC, PESTLE Analysis, Porter's Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leader Profiled |
Bioclinica, Bio-Optronics Inc., DATATRAK International Inc., ERT Clinical, IBM, Medidata Solutions Inc., MedNet Solutions, Inc., Oracle, Parexel International Corporation, ArisGlobal., and Others. |
SEGMENTAL ANALYSIS
By Delivery Mode Insights
Based on the delivery, the web-based CTMS segment was the leader in the global market in 2024 accounted for a major share of the global market and is expected to grow at a healthy CAGR during the forecast period. The rising number of research centers in developed and developing countries majorly propel segmental growth. In addition, increasing investments in developing novel drugs across the globe further boost the segment’s growth rate.
The cloud-based CTMS segment is expected to showcase a promising CAGR during the forecast period due to the increasing use of big-data applications in healthcare. In addition, the rise in the demand to speed up clinical trials and produce vaccines for rare diseases is propelling the segment’s growth rate.
By Component Insights
Based on components, the software segment occupied the largest share of the global CTMS market in 2024, and the segmental domination is likely to continue throughout the forecast period. Factors such as the growing support from the I.T. industries, the prevalence of real-time data tracking, and user-friendly devices contribute to the segmental growth.
The Hardware segment is another lucrative segment and is predicted to account for a notable share of the global market during the forecast period owing to the growing investments from private and public organizations, increasing adoption of the latest technologies in the research centers, and introducing innovative therapeutic procedures.
The services segment is estimated to grow at a robust CAGR of 16.1% during the forecast period, owing to increasing disposable income in urban areas.
By End User Insights
By end-users, the pharmaceutical segment occupied the leading share of the global market in 2024 and is expected to grow at a healthy CAGR during the forecast period, owing to the growing healthcare infrastructure and an increasing number of patient visits to hospitals.
On the other hand, the CRO segment is predicted to showcase domination in the market and witness the fastest CAGR during the forecast period.
By Type of System Insights
Based on the type of system, the licensed enterprise-based segment had the largest share of the global market in 2024 and is expected to continue the same in the coming years to promote quality services. In addition, the growing incidence of chronic diseases like cancer contributes to the segmental growth.
The site-based segment is predicted to grow at the highest CAGR during the forecast period due to the increasing number of pharmaceutical companies in developing countries and ongoing research and development activities.
REGIONAL ANALYSIS
The North American CTMS market accounted for the major share of the global market in 2024. The domination of the North American region in the global market is predicted to continue throughout the forecast period. The presence of a well-established pharmaceutical industry, extensive clinical trial activity, stringent regulatory framework, increasing emphasis on patient safety, rapid adoption of advanced technology, es and a high focus on innovation majorly propel the North American market growth. The U.S. held the leading share of the North American market in 2024, and the same is estimated to continue during the forecast period owing to the availability of skilled research professionals and robust infrastructure, the presence of leading contract research organizations (CROs), and the growing number of collaborations between pharmaceutical companies and academic research institutions. During the forecast period, Canada is predicted to register a healthy CAGR in the North American regional market.
The European CTMS Market accounted for a notable share of the global market in 2024 and the expected to grow at a healthy CAGR during the forecast period. The presence of several leading pharmaceutical and biopharmaceutical companies, rising demand for personalized medicine and targeted therapies, favorable government regulations, supportive research infrastructure and rising emphasis on real-world evidence and post-marketing studies contribute to the growth of the European market. An increasing number of clinical trial networks and international collaborations and growing in digital health and electronic health records (EHR) systems further boost the growth rate of the European market. Germany, France, and the United Kingdom controlled the major share of the European market in 2024.
The CTMS market in the Asia Pacific is predicted to showcase the highest CAGR during the forecast period worldwide. The growing outsourcing of clinical trials to countries like India and China, large patient populations, diverse genetic profiles and lower trial costs drive the CTMS market in APAC. The growing investment in healthcare infrastructure and R&D capabilities, increasing number of contract research organizations (CROs) and clinical trial service providers, rising number of initiatives to attract foreign investment and clinical research, growing prevalence of chronic diseases and increasing demand for innovative therapies further promote the APAC CTMS market growth.
The Latin American CTMS market is predicted to account for a considerable share of the global market during the forecast period owing to factors such as growing prominence as a preferred destination for clinical trials and the benefits of the Latin American region to consider for outsourcing clinical trials such as cost advantages, diverse patient pool, and favorable regulatory environment.
MEA is estimated to grow steadily during the forecast period. Emerging as a hub for clinical trials, particularly in the Middle East. The growing healthcare expenditure, improving research capabilities, increasing number of initiatives from several governments to attract clinical trials and enhance healthcare infrastructure and the availability of treatment-naive patient populations for clinical research contribute to the MEA CTMS market growth.
KEY MARKET PARTICIPANTS
Companies playing a notable role in the global CTMS market are Bioclinica, Bio-Optronics Inc., DATATRAK International Inc., ERT Clinical, IBM, Medidata Solutions Inc., MedNet Solutions, Inc., Oracle, Parexel International Corporation, and ArisGlobal.
RECENT MARKET DEVELOPMENTS
- In March 2020, Mednet announced that it would conduct immunology-related research on COVID-19 to develop a vaccine and treatment procedure. The clinical trial management systems used in this company are competent and flexible for the researchers, which helps them design a product in a pandemic.
- In June 2019, Dassault Systèmes acquired Medidata Solutions, Inc., recognized as the best workplace in the U.K. Medidata is well known for its end-to-end approach to developing a drug. The acquisition has brought many changes in clinical trials and shown excellence in productivity.
- In October 2019, Bio-Optronics, a leading software and services company, introduced clinical management products such as CCeSource, CCeDocs, and CCeConsent, whereas these three products fall under CCTrialSuite. The e-products will reduce the complexity of life sciences and offer clinical needs for hospitals and health systems.
MARKET SEGMENTATION
This research report segmented and sub-segmented the global clinical trial management system market based on delivery mode, component, end-user, type of system, and region.
By Delivery Mode
- Web-based CTMS
- On-premises CTMS
- Cloud-based CTMS
By Component
- Software
- Hardware
- Services
By End User
- Pharmaceuticals
- Contract Research Organization
- Other End Users
By Type of System
- Site-based Licensed Enterprise-based
- Licensed Enterprise-based
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- The Middle East and Africa
Frequently Asked Questions
What is the expected market size of the clinical trial management system market worldwide?
The global clinical trial management system market size is expected to reach USD 2,450 Million by 2033.
Which region holds the biggest market share of the clinical trial management system market?
North America region holds the biggest share of the global clinical trial management system market.
what is the compound annual growth rate (%) of the global clinical trial management system market during the forecast period?
The clinical trial management system market size is forecasted to grow at a CAGR of 12.6% from 2025 to 2033
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]