Global Biologics Safety Testing Market Size, Share, Trends & Growth Forecast Report By Product & Services, Test Type, Application, End-User and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), Industry Analysis From 2025 To 2033.
Global Biologics Safety Testing Market Size
The size of the global biological safety testing market was worth USD 4.63 billion in 2024. The global market is anticipated to grow at a CAGR of 14.13% from 2025 to 2033 and be worth USD 15.21 billion by 2033 from USD 5.28 billion in 2025.

For new product categories, biological safety testing is essential. For example, biological safety testing guarantees that vaccines and biopharmaceuticals are not contaminated and meet safety quality standards. Biopharmaceutical products are used to diagnose, prevent, and treat a wide range of disorders. Biologics are complex compounds made from the genes of live organisms and combined utilizing modern DNA technologies. Biologics are advanced medications used to treat cancer, cardiovascular disease, rheumatoid arthritis, and other diseases. Recombinant proteins, tissues, genes, allergens, cells, blood components, and vaccinations are examples of biological medications made from live organisms.
MARKET DRIVERS
The rising threat of pandemics and viral diseases worldwide is anticipated to boost the global biological safety testing market growth.
Pandemics and communicable diseases have become much more common in recent years. In addition, the pharmaceutical and biotechnology industries' growth and significant R&D expenditure by key companies in the life sciences industry, and an increase in the number of new drug launches in recent years are driving the market. These factors are expected to help boost the worldwide biological safety testing market forward.The norms and legislation very strictly regulate quality control. The microbial contamination of the medicine could be harmful to patients' health and lead to long-term repercussions such as cancer or other health problems. These factors create a lucrative possibility for the market's expansion. Furthermore, rising markets and growing biopharmaceutical outsourcing are likely to present considerable growth opportunities for participants in the global biological safety testing market. Biotechnology-derived products, often known as biologicals, have found their way into many facets of healthcare, including illness diagnosis, prevention, and therapy.However, there are certain possible safety concerns that stem from the production procedures and the products' complex biological and structural properties. As a result, rigorous and systematic biological safety testing is required for these products, which will finally allow for a sufficient safety assessment prior to any clinical inquiry. As a result, the market is likely to grow during the forecast period.
MARKET RESTRAINTS
The lack of qualified professionals to undertake biological safety testing procedures and the high investment costs connected with biological safety cabinets may hinder market growth to some extent. Reluctance to accept the product on the market is also regarded as a barrier to the market's overall expansion. The constraints and regulations regulating the use of UV lights in safety cabinets are also seen as a key challenge to the market's development.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
4.13% |
|
Segments Covered |
By Product & Services, Test Type, Application, End-User, and Region. |
|
Various Analyses Covered |
Global, Regional, and country-level analysis; Segment-Level Analysis, DROC; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape; Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
General Electric Company, Koninklijke Philips N.V., Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Fujifilm Corporation, Samsung, Siemens Healthineers AG, Hitachi, Ltd., ESAOTE SPA, SIUI, and Shenzhen Ruqi Technology Co., Ltd., and Others. |
SEGMENTAL ANALYSIS
By Products & Services Insights
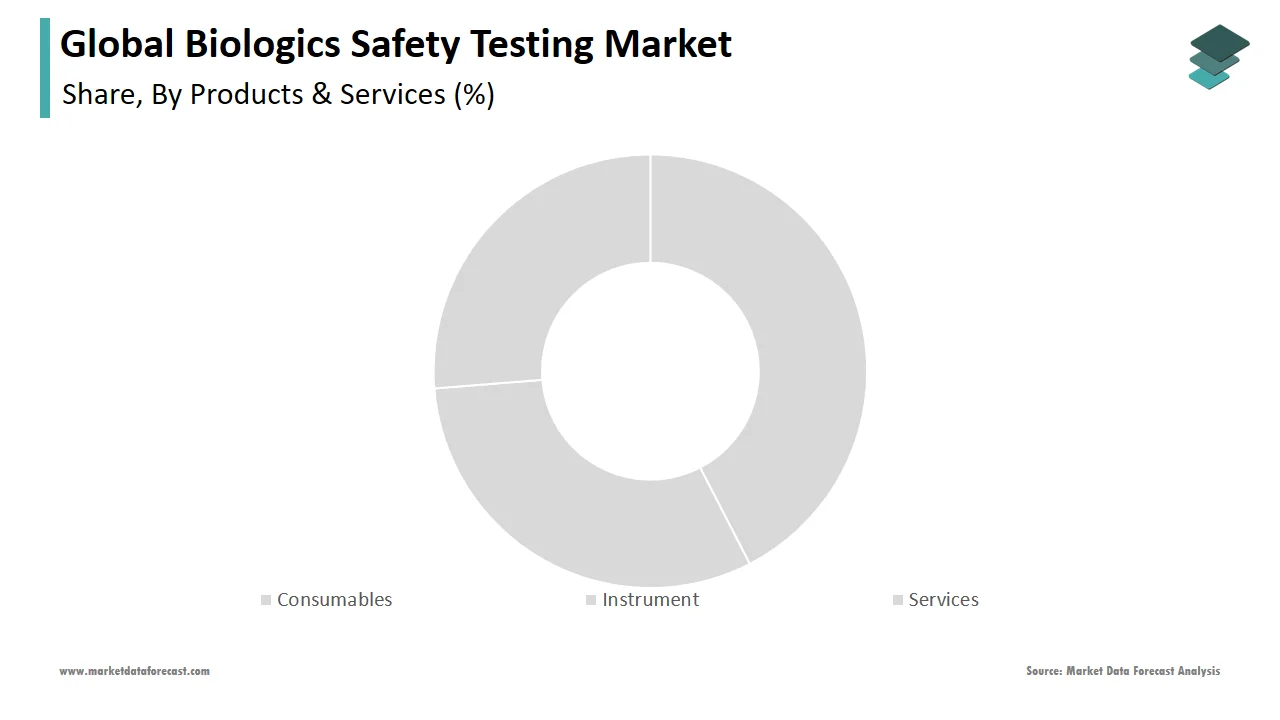
In 2024, the services segment accounted for the largest share of the global biological safety testing market. The large share of this segment can be attributed to biopharmaceutical manufacturers' limited financial resources, capacity constraints, the need to reduce time to market, complex manufacturing requirements, significant investments required to set up manufacturing facilities, and the growing drugs pipeline, all of which have prompted a shift toward outsourcing biologics safety testing to service providers.
By Test Type Insights
Over the forecast period, the bioburden testing segment is expected to hold a considerable proportion of the global biological safety testing market. In addition, organizations such as the World Health Organization have also released guidance for health managers and workers on the infrastructure and standard processes that must be in place for proper sterilization and decontamination of medical devices. These factors are expected to drive up demand for proper sterilization, which will drive up demand for Bioburden testing, which serves as an effective tool in the validation and revalidation of sterilization processes and the assessment of cleaning process efficiency and routine monitoring of manufacturing processes to ensure safety.
By Application Insights
The monoclonal antibody manufacturing segment held the most significant share in the global biological safety testing market during the forecast period due to the growing prevalence of diseases and increased government initiatives to develop monoclonal antibody therapies.
By End-User Insights
Research laboratories include a wide range of capabilities, including drug discovery, cell line development, and cell culture, all of which will necessitate biologics safety testing to provide pathogen-free products and services. In addition, rising academic institutes require installing advanced equipment to comply with biosafety regulations. As a result of the increased focus on research and academia, demand for biological safety testing is expected to increase in the forecast period.
REGIONAL ANALYSIS
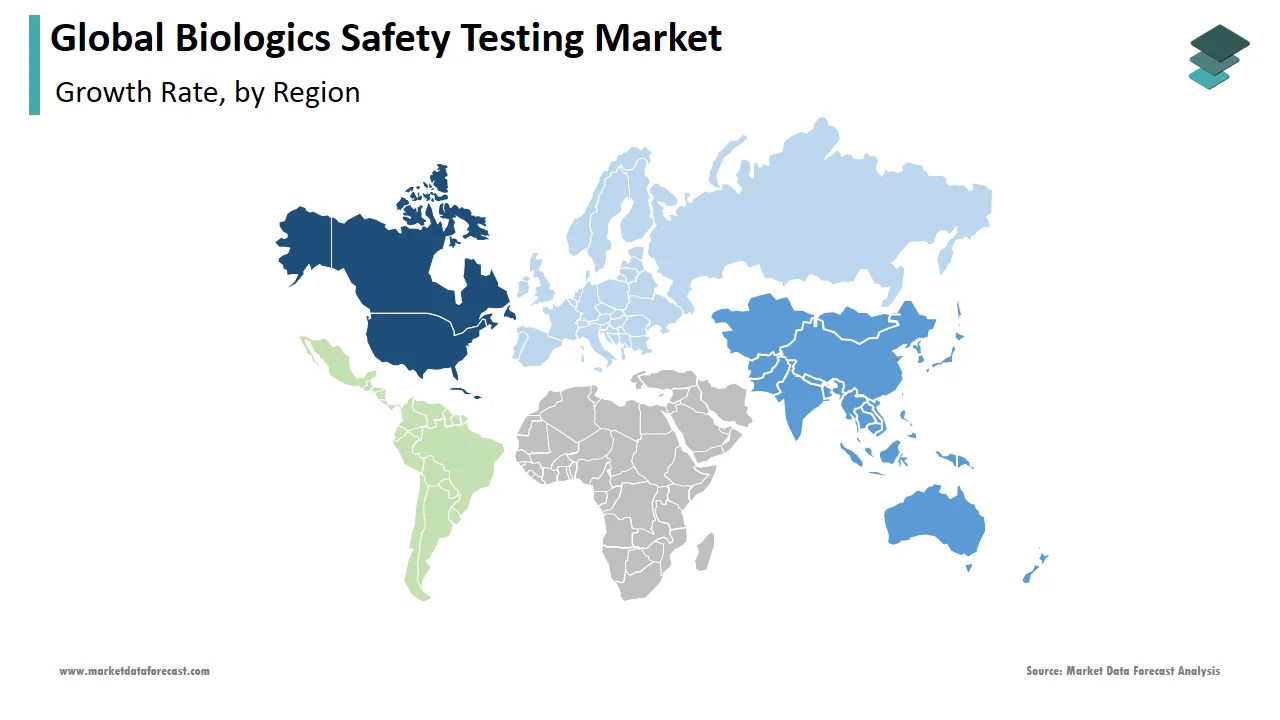
The APAC region is a highly profitable market across the globe. India has taken substantial steps to promote itself as the world's leading major biopharmaceutical innovation hub, including fostering public-private partnerships in biopharmaceutical R&D projects. South Korea is likewise working to establish itself as a global biotechnology powerhouse, focusing on biosimilars, vaccine development, and stem cell therapies. Economic expansion, combined with rising per capita healthcare investment in emerging markets like India, China, and Singapore, is likely to generate significant growth opportunities. Treatment innovations, technological advancements, and expanded healthcare facilities will likely fuel future growth.
The pharmaceutical industry's rapid expansion is a significant driver of the biologics safety testing market in North America. Rising university and government investments, increasing demand for high-quality research tools for data reproducibility, growing consumer awareness about product safety, and the presence of significant market participants in the region are all driving the biologics safety testing market in North America. North America now dominates the market and is projected to continue to do so in the future. The United States is likely to play a significant role in the region's market growth. The increased R&D initiatives connected to COVID-19 due to the outbreak have had a beneficial impact on market growth.
KEY MARKET PLAYERS
Charles River Laboratories International Inc., Merck KGaA, Lonza Group Ltd., Sartorius AG, SGS S.A., Thermo Fisher Scientific Inc., Pace Analytical Services Inc., WuXi AppTec, and Cytovance Biologics Inc. are a few of the notable companies operating in the global biological safety testing market profiled in this report.
RECENT MARKET HAPPENINGS
- Charles River Laboratories, Inc. (US) bought Cognate BioServices in 2021 to expand its cell and gene therapy development, testing, and manufacturing offerings, giving clients an integrated solution from basic research to CGMP production.
- Beckman Coulter Life Sciences introduced an RNA Extraction Kit validated for use in virus research workflows in 2020.
MARKET SEGMENTATION
This report on the global biological safety testing market has been segmented and sub-segmented into products and services, test types, application, end-user, and region.
By Products & Services
- Consumables
- Instrument
- Services
By Test Type
- Sterility Tests
- Bioburden Tests
- Endotoxin Tests
By Application
- Vaccine Manufacturing
- Monoclonal Antibodies Manufacturing
- Cellular & Gene Therapy Products Manufacturing
- Blood and Blood Products Manufacturing
By End-User
- Pharmaceutical and Biotechnology Companies
- Contract Development and Manufacturing Companies
- Research and Academia
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa.
Frequently Asked Questions
What is the current size of the biological safety testing market?
The global biological safety testing market size is expected to be USD 4.63 bn in 2024.
What factors are driving the growth of the biological safety testing market?
The growing demand for biological products, the rising number of drug approvals, and the growing focus on quality control and assurance majorly propel the growth of the biological safety testing market.
Who are the key players in the biological safety testing market?
Merck KGaA, Charles River Laboratories International, Inc., Lonza Group Ltd., SGS SA, Thermo Fisher Scientific Inc., and WuXi AppTec Co., Ltd are some of the notable companies in the biological safety testing market.
What are the emerging trends in the biological safety testing market?
Some of the emerging trends in the biological safety testing market include the adoption of advanced technologies such as next-generation sequencing and CRISPR/Cas9 for testing, the increasing use of in vitro testing methods, and the growing focus on the development of rapid and cost-effective testing methods.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
