Global Bioburden Testing Market Size, Share, Trends & Growth Forecast Report By Product, Application, End-User and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), Industry Analysis From 2025 To 2033.
Global Bioburden Testing Market Size
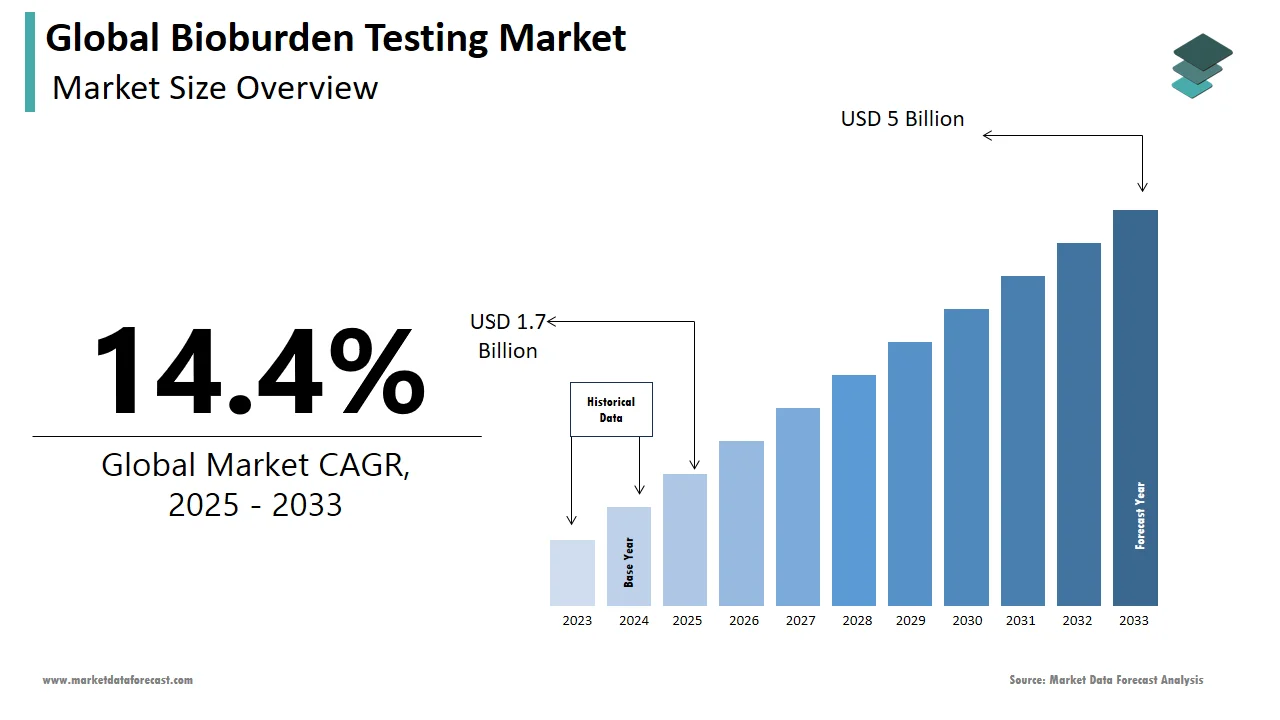
The global bioburden testing market was worth US$ 1.49 billion in 2024 and is anticipated to reach a valuation of US$ 5 billion by 2033 from US$ 1.7 billion in 2025, and it is predicted to register a CAGR of 14.4% during the forecast period 2025-2033.
Bioburden testing involves investigating bacterium and other microorganisms on the surface of medical devices, pharmaceuticals, and other instruments; due to their frequent use, they must be checked thoroughly and sterilized, providing that the testing must be done without fail. In addition, the requirement of a quality check ensures that the drug and other pharmaceutical products have no microorganisms before ingestion. The various processes include removing bacteria and other organisms, one of which is delusion, meaning clearing the bacterium via a solvent. Ultrasonication, Shaking, Vortex mixing, and Flushing Blending is some methods used. The non-elution includes Contact placing, Agar overlay, and Most Probable Number (MPN). The incubation and enumeration include total aerobic and spore-forming colony, as well as total yeast and mold count.
MARKET DRIVERS
Growing usage of medical devices, increased safety concerns connected to food and beverage goods, and a rising number of product recalls due to microbial contamination are propelling the bioburden testing market growth.
Due to the increased awareness of hygiene, pharmaceutical companies, along with Food and beverages companies, are looking forward to better bio-testing methods, one of which accounts for polymerized chain reaction system is expected to become popular in the field of bio-parent testing as the molecular methods help identify pathogens with high precision and detection of various microorganism bacteria fungi virus.
The ever-increasing investment for better research done by academic and economic companies, specifically the rising trend of R&D investments, is anticipating the market's growth. The ever-increasing demand for bioburden testing in contract manufacturing organizations is advancing as these organizations regulate drug manufacturing and its quality. Demand for bioburden testing tends to rise to determine and analyze the amount of contamination and product safety. Instruments and numerical materials used by the public need to ensure a minimum level of bioburden on the external surface as the risk of contamination of certain communication diseases threatens the company's growth. Many organizations are now aware of the importance of bioburden testing.
MARKET RESTRAINTS
The lack of availability of skilled professionals, along with the expensive cost of instruments and lengthy procedures for the approval of specific processes, including a particular type of bioburden testing, is causing a hindrance to the market's growth; as in the US, the cost of Mba production accounts for 100 USD per gram whereas the soaring cost of labor is USD 120 per hour the monoclonal antibiotic drugs follow production challenges. In addition, some restrainers are as filtration can result in an ineffective response against a specific virus.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
14.4% |
|
Segments Covered |
By Products, Application, End Users, and Region. |
|
Various Analyses Covered |
Global, Regional, and country-level analysis; Segment-Level Analysis, DROC; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape; Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
Charles River Laboratories International Inc., Sigma-Aldrich Corporation, SGS S.A., Wuxi Pharmatech (CAYMAN) Inc., Merck & Co. Inc., Becton, Dickinson, and Company, North American Science Associates Inc., Nelson Laboratories Inc., Pacific Biolabs, and ATS Labs Inc., and Others. |
SEGMENTAL ANALYSIS
By Product Insights
Based on the product, the consumables segment is expected to contribute to significant growth as it comprises leading reagent easy to use systems an easily detect microorganisms The toolkits such as Merck KGaA offers Milliflex quantum bioburden testing tool kit aided with I.D. Technology provides consistent results, thus becoming helpful for the pharmaceutical and food industry.
By End-User Insights
Based on the end-user, the pharmaceutical and biotechnology companies segment led the market in 2024. More excellent production of drugs and funding and support through government organizations to the pharmaceutical industry and biotechnology companies, and the increasing production of medical devices along with research outbreaks in fields are providing outcomes for the market growth.
REGIONAL ANALYSIS
North America is dominating the bioburden testing market in terms of share and growth owing to several factors such as R&D investments by pharmaceutical and biotechnology industries along with increasing contamination in Food and beverages and government initiatives related to the accuracy of the safety process of drugs and devices in countries like the U.S. and Canada significantly contribute to the growth of the market.
The market for Asia-Pacific is expected to grow with the fastest CAGR during the forecast period due to the factors like solid investments, increased awareness of the advantages of biological safety testing tools, and rising healthcare costs. Furthermore, the rising expansion of the pharmaceutical and biotechnology industries, demand for better infrastructure in laboratory and clinical research, small-scale and affordable clinical research techniques, and the development of the pharmaceutical and biotechnology industries further promote the bioburden testing market in the Asia-Pacific.
The European region is also anticipated to account for a substantial share of the global market during the forecast period.
KEY MARKET PARTICIPANTS
Charles River Laboratories International Inc., Sigma-Aldrich Corporation, SGS S.A., Wuxi Pharmatech (CAYMAN) Inc., Merck & Co. Inc., Becton, Dickinson, and Company, North American Science Associates Inc., Nelson Laboratories Inc., Pacific Biolabs, and ATS Labs Inc. are some of the prominent companies operating in the global bioburden testing market.
RECENT MARKET HAPPENINGS
- Almac Sciences, a member of the Almac Group, announced the expansion of biologics testing to its existing portfolio of analytical solutions in 2020.
- The new Milliflex Oasis system, available from 2023, is an all-in-one filtration solution that includes sterilized, ready-to-use filtration units and pharmacopeia-compliant media for unparalleled comfort and peace mind when it comes to water and bioburden testing.
- In July 2022, to assist the Indian life science community in developing its microbiological quality control competence, Merck announced the opening of its first Microbiology Application and Training (MAT) Lab in Jigani, Bengaluru. This lab will provide facilities and technical expertise. The MAT Centre is 1,100 square feet and costs €200,000 to build. Sterility testing, quick bioburden testing, pyrogen testing, advanced membrane filtration, and other pharma Q.C. microbiology applications are among the testing services provided in the lab.
MARKET SEGMENTATION
This research report on the global bioburden testing market is segmented and sub-segmented into the following categories.
By Product
- Consumables
- Culture Media and Reagents & Kits
- Other Consumables
- Instruments
- Automated Microbial Identification Systems
- PCR
- Microscopes
- Other Instruments
By Application
- Raw Material Testing
- Medical Devices Testing
- In-process Testing
- Sterilization Validation Testing
By End-User
- Pharmaceutical and Biotechnology Companies
- Medical Device Companies
- Contract Manufacturing Organizations (CMOs)
- Food and Beverages Companies
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Frequently Asked Questions
How much is the global bioburden testing market going to be worth by 2033?
As per our research report, the global bioburden testing market size is estimated to be worth USD 5 billion by 2032.
Which region led the bioburden testing market in 2024?
Geographically, the North American regional market dominated the bioburden testing market in 2024.
Which are the significant players operating in the bioburden testing market?
Companies playing a significant role in the global biourden testing market are Charles River Laboratories International Inc., Sigma-Aldrich Corporation, SGS S.A., Wuxi Pharmatech (CAYMAN) Inc., Merck & Co. Inc., Becton, Dickinson, and Company, North American Science Associates Inc., Nelson Laboratories Inc., Pacific Biolabs, and ATS Labs Inc.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
